Piero Picci, Marco Manfrini, Nicola Fabbri, Marco Gambarotti and Daniel Vanel (eds.)Atlas of Musculoskeletal Tumors and Tumorlike Lesions2014The Rizzoli Case Archive10.1007/978-3-319-01748-8_48
© Springer International Publishing Switzerland 2014
48. Biology of Ewing Sarcoma
(1)
Laboratory of Experimental Oncology, Istituto Ortopedico Rizzoli, Bologna, Italy
Abstract
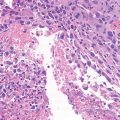
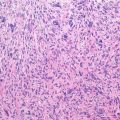
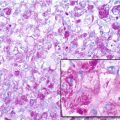
Sarcomas are a heterogeneous group of malignant tumors that are derived from mesenchymal tissues, including bone, muscle, and cartilage. Unlike carcinomas, which affect the elderly and are the final result of a long history of progressively accumulating preneoplastic lesions that allowed the molecular definition of multiple carcinogenic events, mostly sarcomas arise abruptly in infants, and their natural history of sarcomas is still mostly unknown. However, in the last decade, we have gained significant new insights into the genetic abnormalities that underlie the pathogenesis of these tumors. Specific molecular alterations have been associated with specific histological subtypes of sarcomas, leading to a new classification of many sarcomas. Conventionally grouped in either soft tissue or bone sarcomas according to the site of their origin, these tumors can now be genetically distinguished in two main groups: those carrying a tumor-specific recurrent chromosome aberrations that appear to be central to the pathogenesis of the tumor and are therefore included among diagnostic criteria and those with complex karyotypes and variable genetic alterations (Helman and Meltzer 2003; Wunder et al. 2007). Sarcomas with recurrent molecular changes include, among others, Ewing sarcoma family tumors, synovial sarcoma, alveolar rhabdomyosarcoma, myxoid liposarcoma, and myxoid chondrosarcoma. These tumors typically carry disease-specific chromosome translocations that frequently result in the expression of an oncogenic chimeric transcription factor, such as EWS-FLI1 in Ewing sarcoma. EWS-FLI1, which is present in around 85 % of Ewing sarcoma, derives specifically from a chromosomal translocation between chromosomes 11 and 22 and is referred to as t(11;22). While other translocations have also been described in Ewing sarcoma, including t(21;22) and t(7;22), all of the translocations involve the fusion of the EWS gene with an ETS family gene. Survival rates of patients with the different translocations appear to be the same (Le Deley et al. 2010; van Doorninck et al. 2010). Forced expression of EWS-FLI1 in normal cells can induce tumorigenesis. However, the effects of EWS-FLI1 expression are strongly dependent on cellular background (Kovar 2005). For example, EWS-FLI1 transforms immortalized murine NIH3T3 fibroblasts or mesenchymal stem cells and is required for the oncogenic phenotype of patient-derived EWS cells, but its introduction of into primary human or murine fibroblasts leads to growth arrest or cell death, respectively. These data suggest that oncogenic transformation by EWS-FLI requires a permissive cellular background. The critical factors in the permissive background are largely unknown, but may include disruption of the p53 and RB pathways and the presence of an intact IGF pathway as well as of CD99, a 32 kD integral membrane glycoprotein that is highly expressed in most cases of Ewing sarcoma. Recently it was clearly shown how EWS-FLI1 can induce upregulation of IGF1, inducing autocrine activation of IGF-1R and/or of CD99, thus sustaining its transforming activity in mesenchymal stem cells (Cironi et al. 2008; Riggi et al. 2005; Herrero-Martín et al. 2009; McKinsey et al. 2011).
Sarcomas are a heterogeneous group of malignant tumors that are derived from mesenchymal tissues, including bone, muscle, and cartilage. Unlike carcinomas, which affect the elderly and are the final result of a long history of progressively accumulating preneoplastic lesions that allowed the molecular definition of multiple carcinogenic events, mostly sarcomas arise abruptly in infants, and their natural history of sarcomas is still mostly unknown. However, in the last decade, we have gained significant new insights into the genetic abnormalities that underlie the pathogenesis of these tumors. Specific molecular alterations have been associated with specific histological subtypes of sarcomas, leading to a new classification of many sarcomas. Conventionally grouped in either soft tissue or bone sarcomas according to the site of their origin, these tumors can now be genetically distinguished in two main groups: those carrying a tumor-specific recurrent chromosome aberrations that appear to be central to the pathogenesis of the tumor and are therefore included among diagnostic criteria and those with complex karyotypes and variable genetic alterations (Helman and Meltzer 2003; Wunder et al. 2007). Sarcomas with recurrent molecular changes include, among others, Ewing sarcoma family tumors, synovial sarcoma, alveolar rhabdomyosarcoma, myxoid liposarcoma, and myxoid chondrosarcoma. These tumors typically carry disease-specific chromosome translocations that frequently result in the expression of an oncogenic chimeric transcription factor, such as EWS-FLI1 in Ewing sarcoma. EWS-FLI1, which is present in around 85 % of Ewing sarcoma, derives specifically from a chromosomal translocation between chromosomes 11 and 22 and is referred to as t(11;22). While other translocations have also been described in Ewing sarcoma, including t(21;22) and t(7;22), all of the translocations involve the fusion of the EWS gene with an ETS family gene. Survival rates of patients with the different translocations appear to be the same (Le Deley et al. 2010; van Doorninck et al. 2010). Forced expression of EWS-FLI1 in normal cells can induce tumorigenesis. However, the effects of EWS-FLI1 expression are strongly dependent on cellular background (Kovar 2005). For example, EWS-FLI1 transforms immortalized murine NIH3T3 fibroblasts or mesenchymal stem cells and is required for the oncogenic phenotype of patient-derived EWS cells, but its introduction into primary human or murine fibroblasts leads to growth arrest or cell death, respectively. These data suggest that oncogenic transformation by EWS-FLI requires a permissive cellular background. The critical factors in the permissive background are largely unknown, but may include disruption of the p53 and RB pathways and the presence of an intact IGF pathway as well as of CD99, a 32 kD integral membrane glycoprotein that is highly expressed in most cases of Ewing sarcoma. Recently it was clearly shown how EWS-FLI1 can induce upregulation of IGF1, inducing autocrine activation of IGF-1R and/or of CD99, thus sustaining its transforming activity in mesenchymal stem cells (Cironi et al. 2008; Riggi et al. 2005; Herrero-Martín et al. 2009; McKinsey et al. 2011).
The presence of specific chimeric product is very attractive from a therapeutic point of view. Unfortunately the chimeric transcription factors that give rise to Ewing sarcoma are not druggable at the best of our current knowledge. Thus, the most interesting therapeutic options are druggable pathways regulated by EWS-FLI1, such as the IGF-1R-mediated signaling pathway. Antibodies or tyrosine-kinase inhibitors directed against the IGF-1 receptor protein have also been studied as a potential treatment for advanced Ewing sarcoma (Manara et al. 2007) and have implications for therapy. However, phase I–III clinical studies with anti-IGF-IR drugs have clearly indicated modest toxic effects, with mild and reversible hyperglycemia as the most common toxicity, but limited effectiveness. Particularly in Ewing’s sarcoma (EWS), despite the presence of the target in all tumors and ample preclinical evidence supporting the potential value of anti-IGF-IR agents, less than 10 % of cases extraordinarily responded to this therapy (Olmos et al. 2010; Pappo et al. 2011). Evidences for a compensatory role of IR-A when IGF-1R is disrupted (Garofalo et al. 2011, 2012) have been provided, indicating the relationship between these two receptors as one mechanism responsible for acquired and intrinsic resistance to selective anti-IGF-IR therapy. However, further studies are clearly necessary to better define patients that may really benefit from an anti-IGF-IR therapy as well as to rationalize the use of this targeted therapy in combination treatments.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree