Fig. 7.1
Cross section of the mid-upper arm shows contents of anterior and posterior compartments (Reprinted from Anderson et al. [3] with permission)

Fig. 7.2
Cross section of the mid-forearm shows contents of volar and dorsal compartments (Reprinted from Anderson et al. [3] with permission)

Fig. 7.3
(a–c) Cross section of the thigh shows contents and relative positions of anterior, medial, and posterior compartments at these levels. (a) Proximal thigh, (b) mid-thigh, c distal thigh (Reprinted from Anderson et al. [3] with permission)

Fig. 7.4
(a, b) Lower leg. Cross section of the (a) proximal calf and (b) mid-calf shows contents of anterior, deep posterior, posterior, and lateral compartments (Reprinted from Anderson et al. [3] with permission)

Fig. 7.5
Cross section of the foot shows contents of medial, central, and lateral compartments (Reprinted from Anderson et al. [3] with permission)
Generally, the skin and subcutaneous fat, bone, para-osseous spaces, and joint spaces are regarded as a compartment. For the upper extremity, the periclavicular region, axilla, antecubital fossa, wrist, and dorsum of the hand and for the lower extremity the groin, popliteal fossa, ankle, and dorsum of the foot are considered extracompartmental.
7.3 General Rules for Biopsy Safety
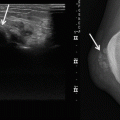
A CT scan with intravenous iodine contrast injection or MRI is performed to localize the lesion precisely and to plan and guide the biopsy [11]. Ultrasound with Doppler and sonoelastography may be used in superficial lesions [7]. The entry point and the pathway are determined, avoiding nerve, vascular, articular, and visceral structures (Fig 7.6).


Fig. 7.6
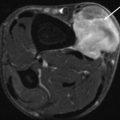
Percutaneous core needle biopsy, with CT guidance, of a deeply seated mass lesion with intralesional calcification. Histologic examination revealed a myositis ossificans. Control examination after conservative therapy showed more typical, zonal calcification of the lesion
General principles for safe percutaneous biopsy include:
- 1.
The shortest path between the skin and the lesion should be chosen.
- 2.
The needle should not traverse an uninvolved compartment.
- 3.
Joint and neurovascular bundles should be avoided.
- 4.
The anticipated needle path should be discussed with the surgeon who will be performing the definitive surgery (Fig.7.7).

Fig. 7.7
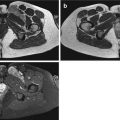
(a–c) Small, pigmented villonodular synovitis (PVNS) in a young female patient at the right popliteal space. (a) MRI of both knees in supine position, axial spin-echo T1-weighted image with fat suppression (FS) and after intravenous gadolinium-DTPA administration. A small, markedly enhancing mass lesion at the left popliteal space adjacent to the popliteal neurovascular structures is seen (white arrow). (b) Computed tomography of the left knee, axial plane in prone position with soft tissue window and level settings and after intravenous administration of iodinated contrast material. The nonenhancing, hypointense mass lesion is seen at the lateral aspect of the enhancing popliteal neurovascular bundle (circle). Entry point in relation to an external radiopaque marker is measured (arrowheads); biopsy pathway (arrow) and skin-to-lesion interval and skin to deepest margin of the lesion (braces) are determined. (c) Computed tomography of the left knee after insertion of biopsy needle, comparable axial plane, verification of needle position before cut
7.4 Diagnostic Accuracy
Percutaneous musculoskeletal biopsy (PMSB) can be performed by fine-needle aspiration biopsy (FNAB), core needle biopsy (CNB), or open (incisional) biopsy. Excisional biopsy should be used only for small lesions (<3 cm) or when the radiologist is convinced that the lesion is benign [7, 10, 20].
It is now generally accepted that in nonhomogeneous lesions, biopsy should be planned to include vital tumor tissue that is characterized by angiogenesis on Doppler ultrasound, contrast enhancement on CT, or MRI and/or nuclear activity on PET imaging. To improve accuracy two or multiple samples might be taken at different locations including morphologic different lesion characteristics.
Open surgical biopsy is advocated by Huvos [19], who claims that only an adequate amount of removed tissue will allow for a maximal diagnostic benefit.
Kilpatrick et al. [23] used fine-needle aspiration biopsy (FNAB) and obtained a histogenetically specific diagnosis in 93 % of cases of pediatric bone and soft tissue tumors, all of which were correctly recognized as either benign or malignant. In adults FNAB is recommended for diagnosis of tumors in the head and neck region [30] and whenever direct incisional biopsy is contraindicated [10, 13]. Gonzalez [13, 14] reported a specificity of FNAB of more than 90 %, the method being most effective when performed by an experienced pathologist. Concordance between FNAB histopathological and cytologic grading in musculoskeletal sarcomas treated by FNAB was studied by Jones et al. Although a statistically significant correlation between cytologically assigned grade and final histopathological grade is found, statistical analysis revealed only a moderate correlation between the two with an overall r value of approximately 0.57. Cytological analysis tended to undergrade in comparison to final histopathological grading. FNAB was moderately successful at predicting histopathological grading. Only analysis of nuclear atypia showed good correlation with final surgical grade [21].
Skrzynski et al. [31] performed a prospective study on the value of closed core needle biopsy (CNB) in 62 patients with soft tissue tumors or bone tumors with soft tissue extension. The diagnostic accuracy was 84 % or 96 %, respectively, for groups of patients who underwent open biopsy performed by the same surgeon. Disadvantages include a nondiagnostic biopsy, indeterminate biopsy, and potential errors in histological grade. The hospital charges for a closed core biopsy were $1106 compared with $7234 for an open biopsy! Comparable results are reported by Hodge [16] with a diagnostic accuracy of 76.9 %.
Bennert [4] evaluated the diagnostic yield of FNAB relative to that of CNB in 117 patients with soft tissue lesions (STL). FNAB was unsatisfactory in 44 patients, 22 of whom were correctly diagnosed with CNB. The author’s conclusion was that FNAB gave a yield identical to that of CNB and that unsatisfactory FNAB should prompt further evaluation by CNB.
Hau et al. [15] studied the accuracy of CT-guided FNAB relative to CNB in a large number of patients (N = 359) with musculoskeletal lesions. They found an overall accuracy of 71 %, i.e., 63 % for FNAB (N = 101) and 74 % for CNB (N = 258). Biopsies of 81 pelvic lesions had a higher rate of diagnostic accuracy (81 %) compared to non-pelvic sites (68 %). The lowest accuracy (61 % and 50 %, respectively) was noted in lesions of the spine and infectious diseases [15].
The best results were reported by Dupuy et al. [9], who performed 176 CNB and 45 FNAB of musculoskeletal neoplasms under CT guidance. They obtained an accuracy of 93 % for CNB and 80 % for FNAB. Complication rate was less than 1 %.
In a recent prospective study on categorizing (malignant–benign) and grading of 18 soft tissue tumors by Kaur et al. by biopsy with FNAB and CNB, FNAB had a categorizing specificity of 90.9 % and CNB a specificity of 100 %. Cytologic grade was concordant with CNB in 81.1 %. They conclude that FNAB and CNB alleviate the need for an open biopsy in diagnosing and grading of musculoskeletal neoplasms [22]. Strauss et al. in a prospective study on 530 patients CNB could differentiate soft tissue sarcomas from benign soft tissue tumors with an accuracy of 97.6 %. High-grade lesions were differentiated from low-grade sarcomas with an accuracy of 86.3 % [32].
Ray-Coquard et al. evaluated CNB in 110 STS patients. They found lower sensitivity in low-grade sarcomas (85 %) compared to high-grade sarcomas (100 %). Histological grading on CNB seems hardly feasible, except for grade III tumors [28]. Didolkar et al. concluded that CNB in malignant MSK lesions had a higher diagnostic yield of 94 % compared to benign lesions with a diagnostic yield of 58 % [8]. This is in contradistinction with a study performed in our institution in which the accuracy of PMSB (bone and soft tissue lesions) was higher in benign lesions (90.2 %) compared to 86 % for malignant lesions. This study performed in our institution in 2012 compared surgical open biopsy, CT-guided CNB, and PET–CT-guided CNB in 149 musculoskeletal lesions (36 soft tissue lesions (STL) and 113 bone lesions (BL)). In PET–CT-guided biopsy, the biopsy was performed during the PET examination at the area in the tumor of most intense radiolabeling with PET–CT-fused images to prove the needle localization (Fig. 7.8). Characterization and grading accuracy was, respectively, 80.5 %/90.2 % for surgical biopsy, 88.2 %/95.6 % for CT-guided biopsy, and 92.5 %/95 % for PET–CT-guided biopsy. For STL the characterization efficacy was 80 % for surgical biopsy, 77.8 % for CT-guided biopsy, and 88.2 % for PET–CT-guided biopsy. In our institution (Belgium), the cost of surgical biopsy ranges from €262 to 380, CT-guided biopsy €159 to 296, and PET–CT-guided biopsy €530 to 667. We concluded that PET–CT-guided biopsy was not cost effective but that PET information, if available, should be used to determine the biopsy location in the specific area of most intense radiolabeling. Surgical biopsy should only be performed in case of inconclusive CT guided [12].