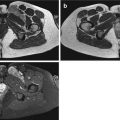
Fig. 14.1
(a–c) Pigmented villonodular bursitis within the pes anserine bursa. (a) Coronal T1-weighted MR image. (b) Coronal T2-weighted MR image. (c) Coronal gradient echo MR image of the left knee. A multilobulated solitary soft tissue mass is demonstrated which is of intermediate to slightly higher SI relative to skeletal muscle on T1-weighted images (a). It is predominantly of low SI on T2-weighted images with effusion within the bursa (b). The low SI is due to its haemosiderin content, which is more conspicuous on gradient echo sequences (c)
The aetiology for TSGCT remains controversial, and several theories have been postulated including a neoplastic process [95], an inflammatory or reactive process, a sequela of trauma and a localised abnormal lipid metabolism [52, 85, 91, 119, 125]. The general consensus is that the disease is either a locally aggressive neoplasm or a reactive synovitis [10]. Chromosomal abnormalities [105], autologous proliferation, high rate of local recurrence [113] and extremely rare cases of metastases favour a neoplastic origin [3, 6, 90, 113, 117]. It has been suggested that the localised form should be considered benign with the diffuse form as aggressive and managed as per malignant lesions. Treatment options for D–TSGCT, therefore, include the use of chemotherapy, radiotherapy and extensive resection, or even limb amputation in some rare circumstances [6, 92]. The new WHO classification groups GCTTS with LNS and D–GCT with PVNS and describes them as one entity as either localised or diffuse-type TSGCT, respectively. As the clinical and imaging presentations vary, we have described these entities separately.
14.1.1.1 Localised Tenosynovial Giant Cell Tumour
Localised TSGCT includes extra-articular GCTTS (nodular tenosynovitis) and intra-articular LNS (synovial giant cell tumour):
Epidemiology:
GCTTS:
GCTTS commonly occurs between 30 and 50 years of age and is rarely seen in those <10 or >60 years of age. Mild female predominance is noted with a 2:1 female-to-male ratio [116]. GCTTS may arise in any synovial sheath. Less commonly, they can arise from synovial lining of a joint or bursa [7]. It has a predilection for the synovium of the tendon sheaths of the hands, particularly the volar surfaces of the fingers. It is the second commonest soft tissue mass of the hand after ganglion cysts. Lesions typically occur near the interphalangeal joints and have been noted to occur more commonly in the first three fingers of the right hand [71, 79]. Less common sites include the ankle/foot, knee, elbow and hip.
LNS:
This reflects a rare entity and, although it shares histological features with PVNS, occurs less frequently. It is, however, important to make a distinction between the two entities as their clinical presentation and treatment differ [71]. LNS represents localised synovial proliferation and is more common in the fifth and sixth decade with no sex predominance [128]. It is distinguished from its diffuse counterpart by being localised to a single compartment within the joint and is almost exclusively seen in the knee [82]. The most common site within the knee is the infrapatellar (Hoffa) fat pad [71] (Fig. 14.2) and less frequently in the suprapatellar recess (Fig. 14.3), intercondylar notch and adjacent to the cruciate ligaments (posterior > anterior) [48, 129]. Other reported sites include the wrist (Fig. 14.4) and ankle (Fig. 14.5).
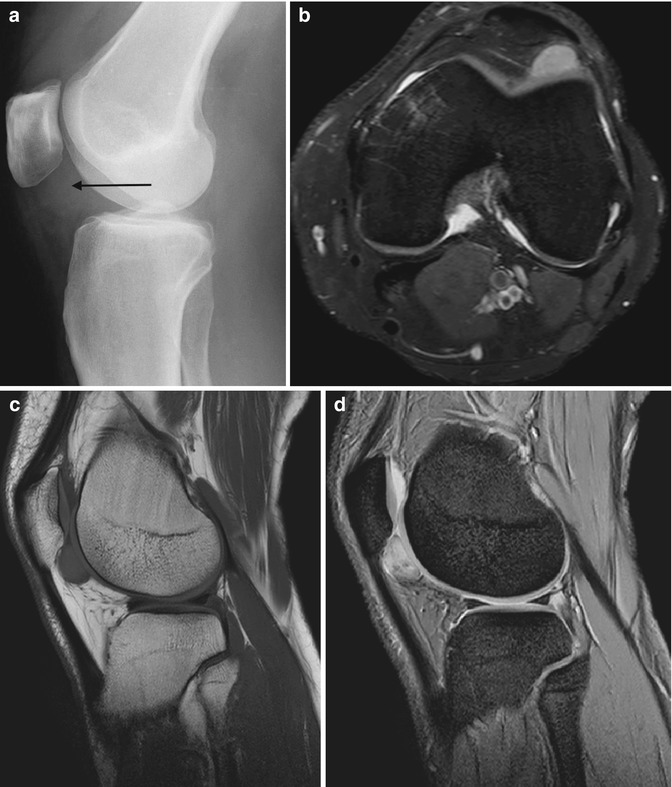
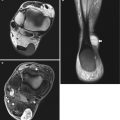
Fig. 14.2
(a–d) Localised form of pigmented villonodular synovitis (PVNS) of the knee within Hoffa’s fat pad, which is the most common location for localised nodular synovitis (LNS). (a) Radiograph of the knee. (b) Axial PDFS MR image. (c) Sagittal T1-weighted MR image. (d) Sagittal gradient echo MR image. Radiograph demonstrates a dense soft tissue nodule (arrow) outlined by the fat within Hoffa’s fat pad (a). LNS is of relatively high SI on fluid-sensitive sequences with variable internal regions of low SI, due to its haemosiderin content (b). It is of intermediate to slightly high SI relative to skeletal muscle on T1-weighted images (c). Haemosiderin is more conspicuous on gradient echo sequences (d). Joint effusion is atypical, unlike its diffuse counterpart, PVNS
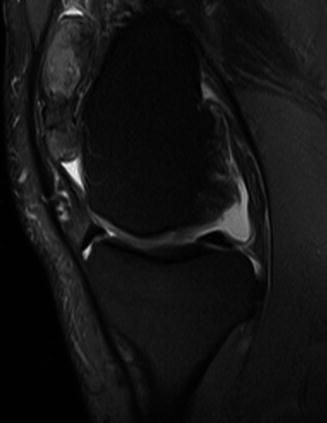
Fig. 14.3
Localised nodular synovitis within the suprapatellar fat pad. Sagittal PDFS MR image demonstrates a predominantly hyperintense mass with intralesional local signal foci, which represent haemosiderin deposits
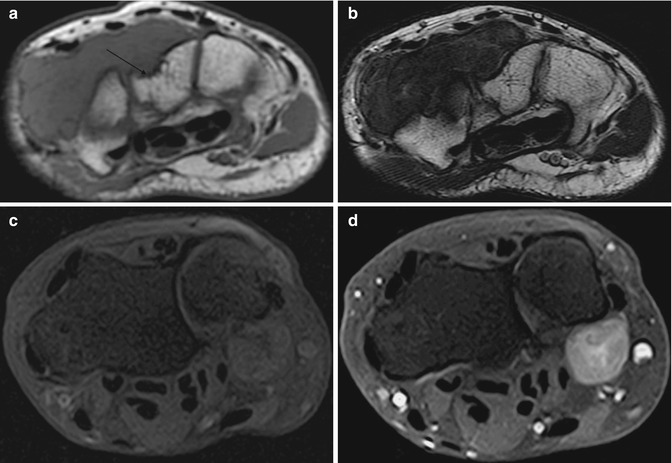
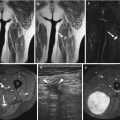
Fig. 14.4
(a–d) Localised nodular synovitis of the wrist in two patients (a–d). (a) Axial T1-weighted MR image. (b) Axial T2-weighted MR image. (c) Axial T1FS pre-gadolinium. (d) Axial T1FS post-gadolinium. A well-defined soft tissue mass that is of isointense SI to skeletal muscle on T1 demonstrates erosion of the dorsal surface of the capitate (arrow) (a). The soft tissue mass is of hypointense SI on T2 weighted, and demonstrates pressure erosion of the capitate and trapezium (b). There is homogeneous enhancement reflecting its vascularity (c, d)
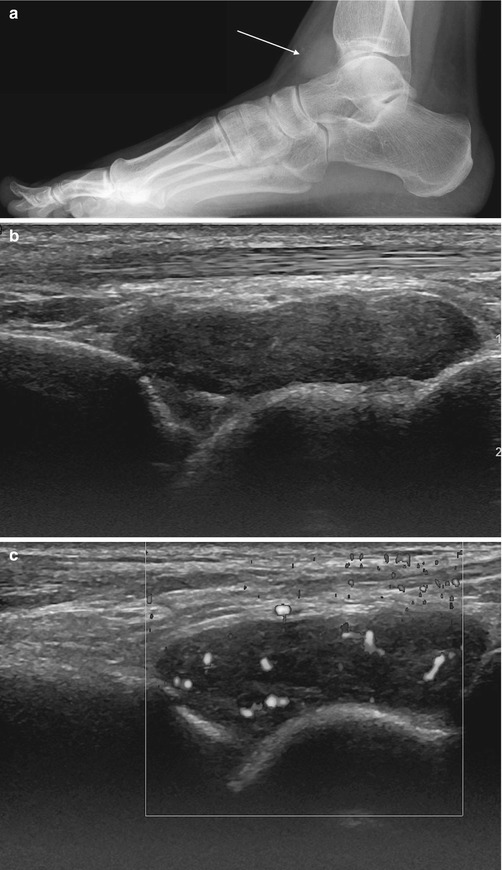
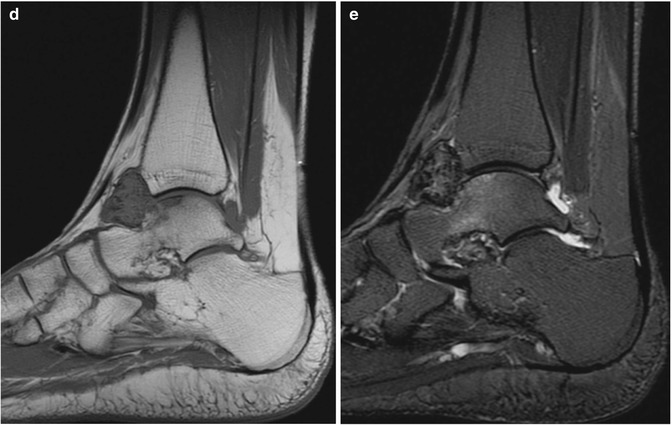
Fig. 14.5
(a–e) Localised nodular synovitis (LNS) of the ankle. (a) Radiograph. (b) Longitudinal ultrasound. (c) Longitudinal power Doppler ultrasound. (d) Sagittal T1-weighted MR image. (e) Sagittal STIR MR image. A dense well-defined soft tissue mass (arrow) is seen in the anterior recess of the tibiotalar joint (a). LNS on ultrasound is seen as a heterogeneous solid intra-articular mass, discrete from the overlying tendon (b), with increased vascularity (c). The mass is of isointense SI relative to skeletal muscle on T1-weighted images with low-signal foci representing its haemosiderin content (d). On fluid-sensitive sequences, the mass is hypointense peripherally with intralesional areas of high SI due to the presence of fat, oedema or inflammation (e)
Clinical behaviour and gross findings:
GCTTS:
This often presents as a slow-growing painless mass. It starts as a nodular synovial lesion projecting into the tendon sheath. Exophytic growth within a restricted anatomical space and pressure effect from the adjacent tendon give rise to its multinodular appearance [71]. Lesions are typically well-circumscribed, firm and rubbery masses surrounded by a fibrous capsule. Grossly, they are often pink-grey lesions with areas of yellow or brown. Lesions are firmly attached to the deep structures and rarely involve the skin. GCTTS infrequently erodes or infiltrates the subjacent bone [115]. Lesions in the hand are usually small (<4 cm) and can become larger in other sites. Treatment is surgical excision, but complete excision can be difficult depending on its extent with reported recurrence rates between 4 and 45 % [54, 64]. Statistically significant risk factors for recurrence include the presence of adjacent degenerative joint disease, location at the distal interphalangeal joint of the finger or interphalangeal joint of the thumb and the radiographic presence of an osseous pressure erosion [98].
LNS:
This entity presents with pain, swelling and typically with locking symptoms, when present in the knee [39, 130]. It is slow growing and not infiltrative. There is no haemorrhagic effusion (Fig. 14.2), unlike PVNS. LNS is typically adherent to the synovium or may have a pedicle extending to it. Cases of torsion [47, 48] or detachment [60] have been reported. LNS is a yellow to brown well-defined mass, without diffuse frond-like projections and less haemosiderin deposition as seen in PVNS [48]. Curative treatment is achieved with surgical excision [48], and, in contrast to PVNS, synovectomy and radiotherapy are not necessary as recurrence is rare [92, 129].
Pathology:
GCTTS:
The microscopic appearance of GCTTS is dependent on the proportion of mononuclear cells, multinucleate giant cells, foamy/lipid-laden macrophages, siderophages and dense hyaline stroma. Osteoclast-like giant cells with variable number of nuclei can also be seen [69]. Haemosiderin deposits are virtually always present [21]. Variable mitotic activity can be demonstrated. Although mitotic figures can occasionally be detected, they are a focal finding and do not indicate malignancy. The histological diagnosis of GCTTS is rarely difficult; however, it can have similarities with other soft tissue tumours, such as fibroma of the tendon sheath, synovial sarcoma and rhabdomyosarcoma [116].
LNS:
Genetics:
The most frequent structural change identified in 30–60 % of TSGCTs is the t(1;2) translocation, which fuses the CSF1 gene on chromosome 1, which encodes for colony-stimulating factor 1, to the collagen 6A3 (COL6A3) gene on chromosome 2 [21, 125]. This results in high levels of CSF1 expression, which attracts macrophages leading to the formation of a tumour-like mass [69, 101]. Furthermore, studies have shown fusion between CSF1 and a yet unidentified gene distinct from COL6A3 [20, 78]. Other authors found trisomies 7 and 5 and a clonal rearrangement of chromosomes 1, 3 and 15 [105]. The CSF1 receptor is a tyrosine kinase receptor, thus is a potential target for biological therapy. Imatinib, which is used in the treatment of renal carcinoma, chronic myeloid leukaemia and gastrointestinal stromal tumours [106], has shown to demonstrate tumour regression in patients with advanced D-GCT [15].
Imaging findings:
GCTTS:
Radiographs are often normal but may demonstrate a well-defined dense soft tissue swelling, thought to be due to its high haemosiderin content (Fig. 14.6a). Approximately, 20 % of cases demonstrate bone erosion. Periosteal reaction, intralesional calcification or cystic degeneration is rarely seen [5, 61].
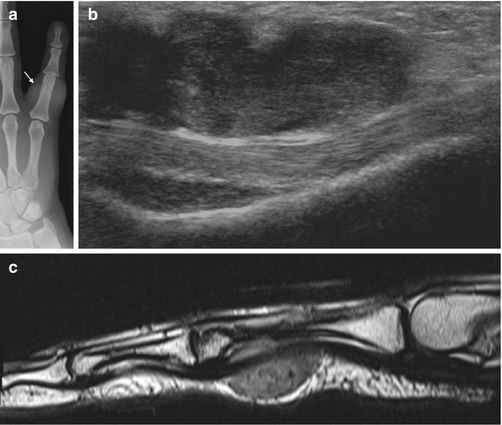
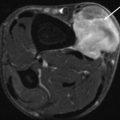
Fig. 14.6
(a–c) Giant cell tumour of the tendon sheath (GCTTS) of the finger. (a) Radiograph of the little finger demonstrates dense soft tissue swelling (arrow) just proximal to the PIPJ. (b) Longitudinal ultrasound of a GCTTS of the little finger demonstrates a multilobulated heterogeneous solid mass related to the flexor tendon. (c) Sagittal T2-weighted MR image shows the typical appearance of a GCTTS, which is seen as a well-circumscribed mass eccentric to or enveloping the tendon
Ultrasound (US) provides precise information regarding the relationship of the tumour with its underlying tendon. Tumours do not move during flexion/extension as the tumour arises from the tendon sheath and not the tendon itself [77]. Lesions are solid and typically lobulated and hypoechoic (Fig. 14.6b); however, hyperechoic and heterogeneous sonographic appearances have been described [77, 120, 122]. GCTTS is typically hypervascular (Fig. 14.7a), and posterior acoustic shadowing can be seen in approximately 33 % of cases [77]. Bone erosions can be detected in approximately 16 % of cases [122]. The main differential for GCTTS in the hand is a ganglion cyst, which can be readily distinguished by its cystic structure [14]. However, ruptured ganglion cysts can appear solid and mimic a GCTTS [77].
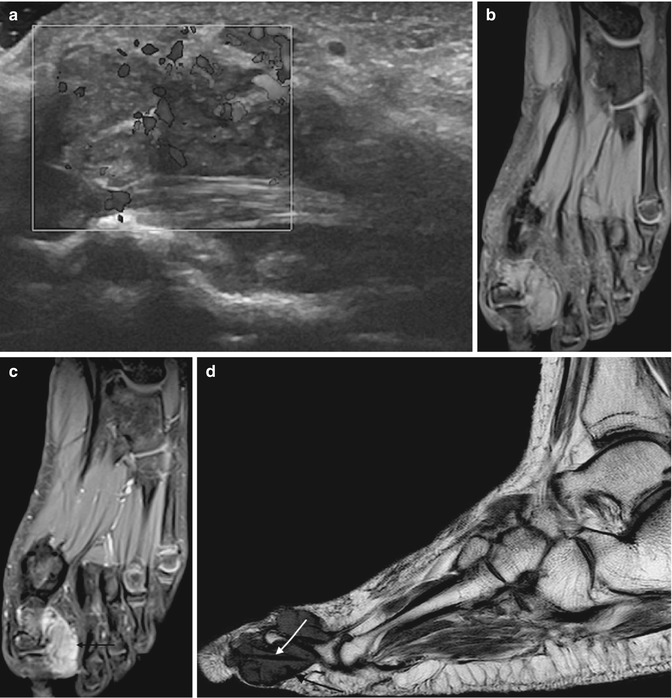
Fig. 14.7
(a–d) Giant cell tumour of the tendon sheath (GCTTS) of the toe. (a) Longitudinal power Doppler ultrasound of a GCTTS of the first toe. GCTTS are hypervascular lesions. (b) Long-axis T1FS pre-gadolinium and (c) long-axis T1FS post-gadolinium. There is homogeneous enhancement of the GCTTS reflecting its vascularity (arrow). (d) Sagittal T1-weighted MR images demonstrates an isointense SI mass relative to skeletal muscle encasing the flexor tendon (white arrow). The mass has a capsule that is typically of low signal (black arrow) secondary to fibrosis or haemosiderin deposits (Colour figure online)
Computer tomography (CT) identifies a dense soft tissue mass related to the tendon and is useful to detect underlying bone erosions or cysts. Contrast enhancement is typically present due to their hypervascular nature [70].
Magnetic resonance imaging (MRI) of GCTTS typically shows a well-circumscribed mass eccentric to or enveloping a tendon (Fig. 14.6c). The capsule is typically of low signal secondary to fibrosis or haemosiderin deposits (Fig. 14.7d) [55]. Tendon sheath effusion is an uncommon finding. The signal characteristics of GCTTS reflect its histological composition, namely, the presence of haemosiderin-laden tissue. The paramagnetic effect of haemosiderin shortens T1 and T2 relaxation times, resulting in low signal intensity (SI) on T1- and T2-weighted spin echo sequences (Fig. 14.7d) [22]. ‘Blooming artefact’ is seen on gradient echo (susceptibility) sequences [10]. Collagenous proliferation also contributes to the characteristically low SI [22, 55, 61]. Intralesional fat signal may be present due to the presence of foamy macrophages. As GCTTS is a vascular lesion, enhancement is seen post-contrast administration (Fig. 14.7c) [22]. MRI is useful in the detection of osseous erosions, though less sensitive than CT. There have been cases that have demonstrated restricted diffusion on diffusion-weighted imaging [83].
Although not generally used as initial staging investigations, bone scintigraphy has been used to localise primary and recurrent GCTTS [62]. It is thought that the hypervascularity and hypercellularity in close proximity to a joint are responsible for increased radionuclide uptake [82] of 99m Tc–MDP [62], 99m Tc–DMSA [11, 62], 67 Ga, 201 Tl [73] and 18F-fluorodeoxyglucose positron emission tomography-computed tomography ( 18 F–FDG PET–CT) [26]. Furthermore, 18 F–FDG PET–CT has been shown to detect recurrent disease [110]. Given its tendency to occur in the extremities, GCTTS should be considered in the differential diagnosis of a lesion with 201Tl activity in the hands and feet [73].
LNS:
Generally, no radiographic abnormality is demonstrated on radiographs. Within the fat pad, LNS may be outlined by fat demonstrating a dense soft tissue nodule (Figs. 14.2a and 14.5a). The increased density seen is related to the iron content. Lesions do not demonstrate calcification, and its presence is suggestive of an alternative diagnosis [70].
US demonstrates a focal heterogeneous echogenic soft tissue mass with increased Doppler signal (Fig. 14.5d–e). In contrast to PVNS, no significant joint effusion is seen.
CT findings are similar to radiographs, demonstrating an intra-articular rounded dense soft tissue mass.
MRI is useful for pre-surgical planning. LNS can present as a well-defined, small ovoid lesion (Fig. 14.2) or as a large multilobulated solitary intra-articular soft tissue mass. LNS is of intermediate to slightly higher SI relative to skeletal muscle on T1-weighted images. It is relatively of high SI on fluid-sensitive sequences with variable circular internal regions of low SI, due to its haemosiderin content. This is more conspicuous on gradient echo sequences (Fig. 14.2a–d). Linear or cleft-like high signal internal regions may be present thought to reflect tissue necrosis [48]. Joint effusion is atypical, and there is moderate to marked inhomogeneous enhancement post-contrast (Fig. 14.4c–d). This is thought secondary to the proliferative capillaries in the collagenous stroma [22]. Osseous erosion may be seen if LNS occurs within confined spaces, such as the wrist (Fig. 14.4a–b).
Differential diagnosis:
GCTTS:
GCTTS closely resembles a fibroma of the tendon sheath (FTS) on MRI. GCTTS is more common by 2.7:1 [35]. Furthermore, FTS is extremely uncommon in the lower extremities; thus, a lower extremity lesion is more likely to be a GCTTS rather than a FTS [35]. Tophaceous gout can mimic GCTTS if localised to the tendon sheath. Generally, soft tissue tumours and tumour-like lesions affecting the extremities, such as ganglion cysts, nerve sheath tumours, foreign body granulomas and synovial sarcomas, display high SI on T2-weighted images. This is in contrast to the low/intermediate SI seen in GCTTS. However, extra-abdominal desmoid tumours [23] and sarcomas, such as malignant fibrous histiocytoma (now termed undifferentiated pleomorphic sarcoma), may display intermediate SI on T2 weighting due to high collagen content. The relevant clinical context can help identify haemosiderin deposition from haemophilic arthropathy, mineralised lesions and foreign bodies, which may be seen as a low-signal mass on both T1- and T2-weighted MR images.
LNS:
Statistically, PVNS is more common than LNS [39], but is more diffuse and demonstrates greater ‘blooming artefact’ due to greater haemosiderin deposits. The differential diagnoses of a mass in the infrapatellar fat pad are myriad. Hoffa’s impingement is an entity characterised by ill-defined inflammation, oedema and fibrosis of the superolateral infrapatellar fat. Intra-articular chondroma of the infrapatellar fat pad has a SI pattern consistent with either cartilage matrix or bone marrow and lacks the deposition of haemosiderin. Other lesions, such as a tophus from gout, do not typically have the same imaging characteristics as LNS and maybe associated with erosions.
14.1.1.2 Diffuse-Type Tenosynovial Giant Cell Tumour
D–TSGCT encompasses D–GCT and its intra-articular analogue PVNS. D–GCT/PVNS is defined as a benign, locally aggressive, proliferative neoplasm.
Get Clinical Tree app for offline access

Epidemiology:
D–GCT:
A rare extra-articular fibrohistiocytic tumour presents as a periarticular soft tissue mass but can be intramuscular or subcutaneous [33]. It affects slightly younger adults than GCTTS, typically <40 years with a slight female predominance [82]. D–GCT has a similar location distribution and symptoms as PVNS.
PVNS:
A rare intra-articular fibrohistiocytic tumour with an incidence of two patients per million. Age and sex distribution is the same as D–GCT. PVNS occurs in large joints and is typically monoarticular. Approximately 80 % occur in the knee, followed by the hip, ankle, shoulder and elbow [71]. Rare cases of involvement of the temporomandibular joint [49] and the spine [118, 121] have been reported. Malignant variants, malignant transformation, and metastases have been rarely reported [3, 6, 19].
Clinical behaviour and gross findings:
D–GCT and PVNS have similar clinical histopathological characteristics. Presentation includes insidious onset of pain and joint swelling which leads to limited range of motion of the affected joint. As PVNS becomes more advanced, the synovial mass constricts the joint, whereas LNS tends to grow outwards, becoming pedunculated. Erosions and subchondral cysts of the adjacent bone are common. The abnormal synovium is prone to haemorrhage and a large joint effusion is often present. The effusion is often recurrent, haemorrhagic and out of proportion to the degree of mild discomfort. D–GCT is a firm multinodular mass of variegated colour with alterations of white, yellowish and brown areas. D–GCT contains numerous capillary fronds and nodular areas. PVNS is a firm red-brown or tan mass with a prominent villonodular growth pattern [81]. Despite being a benign entity, treatment with total synovectomy is recommended, although malignant transformation and metastasis have been reported [6]. External beam radiotherapy can be used as a primary treatment for unresectable cases or as adjuvant therapy for incompletely excised tumours. Intra-articular injection of yttrium-90 (90Y)-labelled colloid (radiosynovectomy) has been used as a local adjuvant after synovectomy [118]. As D–GCT/PVNS is locally aggressive, a high rate of recurrence has been reported. Recurrence can occur in 33–50 % of cases, often with multiple recurrences [89, 112].
Pathology:
Microscopically, D–GCT/PVNS demonstrates histological similarities to the GCTTS. Synovial-like mononuclear cells with foam cells, multinucleated giant cells, inflammatory cells and siderophages are present. However, fewer giant cells are seen than in GCTTS. Osteogenic giant cells are common in GCTTS, but absent or rare in D–GCT. Larger mononuclear cells may have a peripheral haemosiderin rim in the cytoplasm. Cleft-like spaces and blood-filled spaces devoid of giant cells are seen in approximately 10 % of cases of D–GCT. PVNS differs in that it is unencapsulated and has pronounced villonodular architecture with elongated synovial-lined spaces. The occasional predominance of the large histiocyte-like cells can make the diagnosis of D–GCT challenging and can be mistaken for a sarcoma. Mitotic rates up to >20 mitoses per 10 high-power fields (HPF) can be seen.
Genetics:
See section ‘genetics’ in Sect. 14.1.1.1.
Imaging findings:
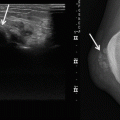
Radiographs are often not diagnostic. With advanced disease, there may be evidence of soft tissue swelling, loss of joint space and periarticular erosion of the bone. The periarticular erosions are usually present on both sides of the joint and are more notable in joints with a tight capsule, such as the hips (90 % of cases) and shoulder (70 % of cases). The erosions tend to be circumferential and produce a classical ‘apple core’, but this can be seen with other synovial-based pathologies (e.g. synovial osteochondromatosis). Erosions are less common in capacious joints such as the knee (25 % of the cases) [10, 71]. These erosive changes show well-defined sclerotic borders and are most likely due to a pressure phenomenon (Fig. 14.8). Interestingly, recent studies suggest the release of a proteolytic enzymes leading to the erosions [87]. The joint space and cartilage are preserved until late in the disease. This and the lack of new bone formation help distinguish PVNS from osteoarthritis. Erosions are typically multiple; however, solitary lesions may mimic a primary osteolytic bone tumour [57]. The absence of periarticular osteopenia helps distinguish D–GCT from an inflammatory synovitis. With PVNS, a dense juxta-articular mass with joint effusion may be seen (Figs. 14.9 and 14.10a). Calcifications are rare in PVNS, and their presence is suggestive of an alternative diagnosis, such as synovial osteochondromatosis.
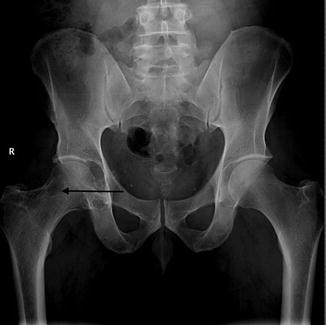
Fig. 14.8
PVNS of the right hip. Radiograph of the right hip, demonstrating well-defined osseous erosions with sclerotic borders (arrow). Erosions seen on both sides of a joint are typical of PVNS
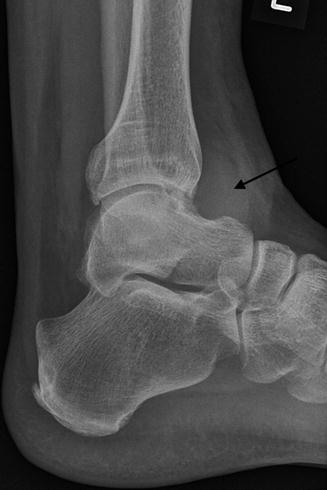
Fig. 14.9
Radiograph of the ankle demonstrating increased juxta-articular soft tissue density (arrow) seen with PVNS
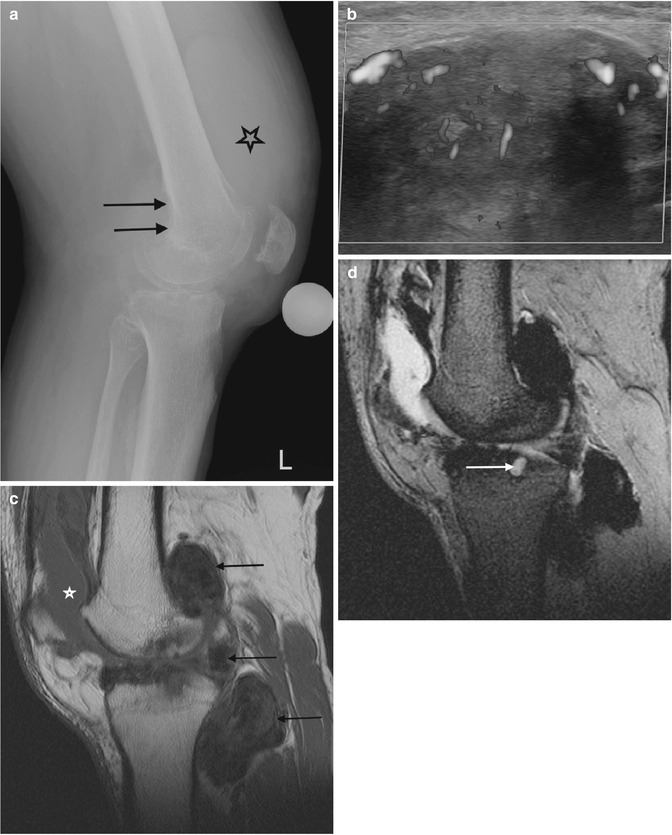
Fig. 14.10
(a–d) Pigmented villonodular synovitis (PVNS) of the knee. (a) Radiograph. (b) Transverse Doppler ultrasound. (c). Sagittal T1-weighted MR image. (d) Sagittal gradient echo MR image. The radiograph (a) demonstrates an increased density within the suprapatellar pouch with evidence of erosive changes of the posterior femoral condyles (arrows). Increased vascularity is typically seen with PVNS (b). In (c), a large joint effusion (star) can be seen, with multiple low T1 SI masses, predominantly in the posterior joint recess (arrows). The low SI is secondary to the haemosiderin content, which demonstrates susceptibility artefact on gradient echo images (d). Erosion and cyst formation of the tibial plateau are noted in (d) (white arrow) (Colour figure online)
On US, D–GCT is a solid periarticular hypoechoic mass with well-defined margins. There is an absence of posterior acoustic enhancement and evidence of internal echoes and increased Doppler signal. Features of an intra-articular proliferative synovitis are seen with PVNS. Typically, there is a large joint effusion with a thickened synovium and a vascular heterogeneous mass (Fig. 14.10b). Occasionally, bone erosion may be detected.
On CT, D–GCT is a non-specific periarticular mass and PVNS is seen as a high-density intra-articular mass. High-attenuation tissue within the joint can also be caused by haemophilia, chronic bleeding due to another cause or calcification. Like GCTTS, D–GCT/PVNS is hypervascular and demonstrates enhancement post-contrast. CT is particularly useful in the assessment of cyst formation and bone erosions [10]. An associated intraosseous soft tissue mass, which extends with a narrow pedicle to the synovium, can be demonstrated [56].
MRI of D–GCT/PVNS reflects its histopathological composition. Though not specific, it is characteristically of low SI on T1- and T2-weighted sequences due to the presence of haemosiderin (Fig. 14.10c) [82], which is more conspicuous on gradient echo sequences due to the ‘blooming artefact’ (Fig. 14.10d). Foci of high T1 SI may be present due to lipid-laden macrophages, and fluid-sensitive sequences can vary in signal depending on the amount of blood, fibrous tissue and oedema. Marked enhancement of the synovitis component is seen post-intravenous gadolinium (Fig. 14.11). Capsular defects can result in juxta-articular ligament spread. MRI is the optimal modality for preoperative assessment to determine tumour size, extent and relationship with adjacent joints (Fig. 14.12). D–GCT is seen as a periarticular mass with the above-described MRI characteristics (Fig. 14.13).
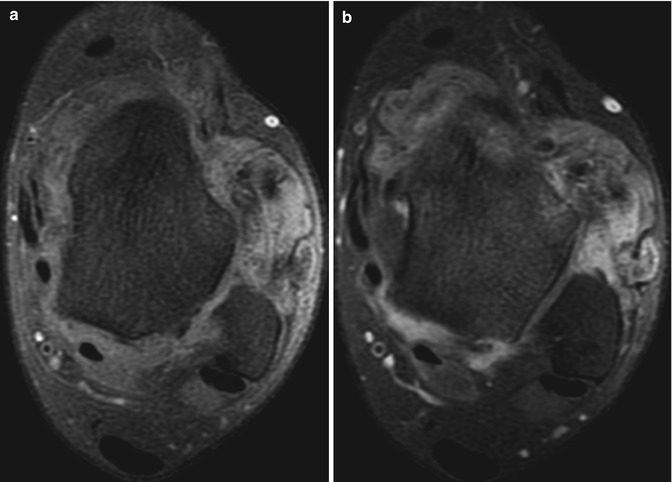
Fig. 14.11
(a–b) Pigmented villonodular synovitis (PVNS) of the ankle. (a) Axial pre-contrast T1FS MR image. (b) Axial post-contrast T1FS MR image. Marked contrast enhancement of PVNS post-gadolinium is seen
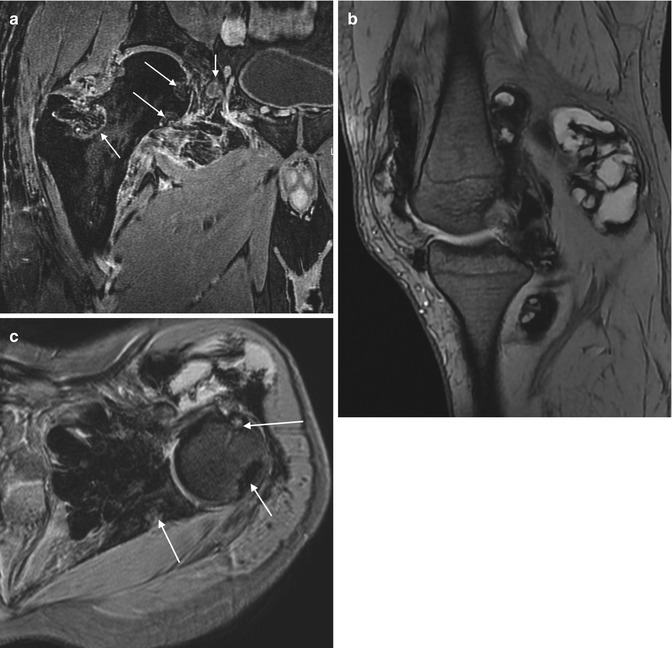
Fig. 14.12
(a–c) Three examples of the ‘blooming artefact’ seen with pigmented villonodular synovitis. (a) Coronal gradient echo MR image of the hip. (b) Sagittal gradient echo MR image of the knee. (c) Axial gradient echo MR image of the shoulder. The presence of haemosiderin causes local changes in susceptibility and therefore loss of MR signal, most conspicuous on gradient echo sequences. Erosions (arrows) secondary to intraosseous extension are often seen, particularly in joints with tight capsules such as the hip (a) and shoulder (c)
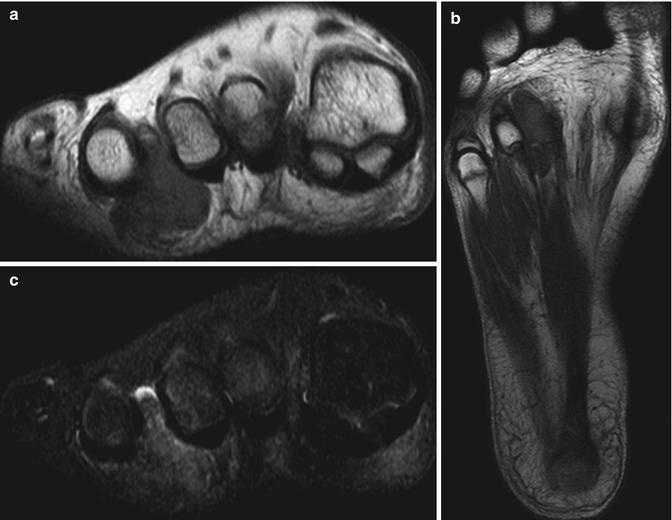
Fig. 14.13
(a–c) Diffuse giant cell tumour of the foot. (a) Short-axis and (b) long-axis T1-weighted MR images. (c) Short-axis STIR MR image. There is a subcutaneous, extra-articular, lobulated mass, which is of intermediate SI relative to skeletal muscle on T1-weighted images with a few foci of low signal in keeping with haemosiderin deposits (a, b). These are more conspicuous on gradient echo sequences due to ‘blooming’ artefacts (not shown). The mass is of high SI on fluid-sensitive sequences in keeping with oedema and inflammation (c). On imaging alone, this lesion could also represent a giant cell tumour of the tendon sheath (GCTTS); however, it is not as intimately related to the flexor tendon as would be expected for a GCTTS
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree