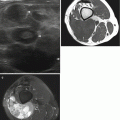
Fig. 1.1
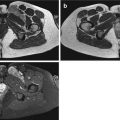
Myxofibrosarcoma in a 65-year-old female patient with painless firm mass at the right thigh. Demonstration of tumoral mass with nonspecific US characteristics for which further MRI examination and subsequent biopsy is mandatory. (a) Sagittal EVOFS US image demonstrating a nonhomogeneous hypoechogenic polynodular lesion centered and with a broad base at the superficial fascia of the rectus femoris, protruding into the subcutaneous tissue, maximal longitudinal diameter 3 cm (longest oblique diameter 5 cm). Indistinct borders with infiltrative aspect of the subcutaneous tissue. (b) Sagittal CDUS image demonstrating marked nonhomogeneous angiogenesis with areas of absent vessels. (c) Axial TSE T2-WI, nonhomogeneous intermediate to low SI lesion with polynodular morphology and stranding at the subcutaneous tissue. (d) Axial TSE T1-WI with FS after IV gadolinium administration. Marked nonhomogeneous enhancement of the lesion and the perilesional stranding. Enhancement of the superficial fascia of the rectus femoris muscle and the vastus lateralis muscle, focal invasion at the level of the rectus femoris muscle (arrow) confirmed after resection
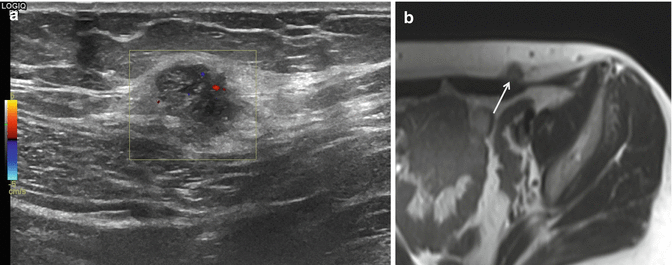
Fig. 1.2
Endometrioma at the abdominal wall in 40-year-old female patient with cyclic painful small mass at the left iliac fossa and previous history of laparoscopic surgery. (a) US examination demonstrating nonhomogeneous hypoechogenic small mass lesion demonstrating small vessels, irregular margins with infiltrative aspect in the subcutaneous tissue and at the rectus abdominis fascia. (b) MRI axial haste T2-WI with nonhomogeneous low to intermediate SI mass superficially located. Note also infiltration of the superficial muscle fascia of the rectus abdominis muscle

Fig. 1.3
Myxoma at the left gluteus maximus muscle in a 60-year-old female patient presenting with painless mass lesion. (a) US examination reveals homogeneous asonant round to oval mass lesion with sharp margins and acoustic retro-enhancement. Maximal diameter 3.5 cm. (b) The lesion is avascular on CDUS examination

Fig. 1.4
Wood fragment at the subcutaneous tissue of the dorsum of the right foot surrounded with cellulitis area. US demonstrates reflective triangular wood fragment (arrow) surrounded with hyporeflective hypervascular tissue
US with CDUS criteria that suggest malignancy are nonhomogeneous echotexture and architectural distortion due to infiltration of adjacent structures with angiogenesis with variable caliber of tumor vessels (Fig. 1.1). Benign tumors are more often homogeneous and regularly delineated and cause displacement rather than invasion of adjacent structures [56, 87]. However, there is considerable overlap between these two groups. Benign tumors such as skeletal muscle vascular malformation (formerly known as hemangioma), neurofibroma, and schwannoma can present with features of poor delineation and nonhomogeneous echotexture, while sarcomas on the other hand often demonstrate sharply defined margins due to pseudo-capsule formation [28]. If these sarcomas are small in size at the time of detection, the tumor necrosis that would result in nonhomogeneity on US may not yet have occurred. Some lesions are, however, also characterized by their specific location, e.g., subungual glomus tumor and branchial cyst [45], adding to the specificity of the diagnosis. CDUS and spectral Doppler analysis of soft tissue tumors is of limited value when differentiating benign from malignant tumors. If a multitubular-organized vascular pattern is present, the tumor is more likely to be benign. Flow characteristics are not specific enough to be applicable in clinical practice [54]. Resistive indices cannot be used to distinguish benign from malignant musculoskeletal soft tissue masses [71].
Experience with the potential value of strain and shear wave elastography in soft tissue tumors is still preliminary [86]. The mean strain ratios in strain elastography of malignant tumors were significantly higher than the mean strain ratios of benign tumors. There was no significant difference for strain histograms and visual scoring. Strain ratios may be used as an additional tool in soft tissue tumor evaluation, possibly minimizing the number of biopsies [108]. Quantitatively and qualitatively, there is no statistically significant association between shear wave velocity and malignancy. Currently, there is no clear additional role compared to B-mode imaging [98].
The best practical approach is to use ultrasound as a tool for selection of those lesions that are confidently diagnosed as benign. To prevent delay in diagnosis, all indetermined lesions should be submitted to further diagnostic work-up by MRI and imaging-guided CNB in that order [16]. Ultrasonographically definite benign lesions are homogeneous cystic lesions with strong posterior enhancement and sharp margins that do not show any vascular signal with CDUS (at highest sensitivity presets). Careful US examination combined with clinical correlation may suggest a specific diagnosis in the case of an epidermal inclusion cyst (Fig. 1.5), lipoma (Figs. 1.21 and 1.22), or, in the presence of a phlebolith, a skeletal muscle vascular malformation [87, 113]. Also in patients with an initial diagnosis of trauma with Morel-Lavallée, hematoma, or muscle tear, it is evident that an accurate history report is important. A hematoma does not arise spontaneously, except in patients with a coagulation disorder or treated with anticoagulation medication. Hematoma does not keep on growing and has the history of a direct or contusional trauma, mostly severe enough.


Fig. 1.5
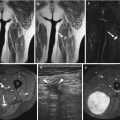
US of the normal skin plantar area (a), heel (b), and nail bed first toe (c), high resolution with differentiation of the epidermis and dermis, for comparison 8–18 mHz 2-cm-wide linear probe and 6–15 mHz 5-cm-wide linear probe (GE healthcare Logic E9 with resp. ML 6–15 and L8-18i). (a) Plantar skin area at 18 mHz. Thin hyperreflective epidermis layer (1) with reflective dermal layer (2) and hyporeflective subcutaneous fat layer (3). (b) Heel area at 11 mHz. Thin hyperreflective epidermis layer (1) with reflective dermal layer (2) and hyporeflective subcutaneous fat layer (3). (c) Nail toe 1. At the nail bed, hypoechoic nail bed and matrix area (arrows) is covered with reflective nail superficially and reflective corticalis of the distal phalanx
The trade-off for high-frequency, linear, musculoskeletal transducers is their limited depth of penetration and the small, static scan field. This is a disadvantage if the soft tissue swelling is large, localized deep in the flexor compartment of the calf, the proximal thigh, buttocks, or trunk or in case of obese patients. Extended field of view sonography (EFOVS) overcomes the disadvantage of a limited, standard field of view [131]. By generating a panoramic EFOVS image, size and anatomical spatial relationships of a soft tissue mass are better evaluated (Fig. 1.1a). EFOVS improves also communication of imaging findings to the referring clinician and contributes to increased reproducibility, which is important for follow-up of lesions [6, 26, 84, 132]. If EFOVS is unavailable, US is not the preferred imaging modality due to its lack of overview and penetration, and MRI should be used as examination of choice [5]. These characteristics of high-frequency transducer characteristics turn into benefits, however, when it comes to diagnosing very small lesions superficially located, lesions as is the case in the wrist, hand, and foot, and lesions of the skin and of peripheral neural origin. The scanning plane can be easily adjusted to the complex local anatomy of the hand, wrist, and foot as was discussed [42, 64, 138].
Other applications of US in soft tissue tumor imaging are US-guided interventional procedures, staging and grading of dermatologic lesions. Diagnostic procedures and therapeutic interventions that are guided by US are gaining in popularity in the musculoskeletal imaging, following the already more established use of this technique in mammography and the abdominal-genitourinary field.
Percutaneous interventions range from ganglion aspiration (18–22 gauge), fine-needle aspiration biopsy (FNAB) in suspected carcinoma metastasis, local recurrence of a soft tissue sarcoma, core needle biopsy (CNB) of extra- and intra-articular solid soft tissue masses, and preoperative needle wire localization of nonpalpable solid soft tissue and vascular tumors to aspiration and culture sampling of a fluid collection, percutaneous catheter drainage of subperiosteal abscess, and muscle biopsy in neuromuscular disease [14, 15, 20, 22, 25, 85, 101, 109, 141]. The procedures can be performed after US selection of the approach (site, depth, and needle angulation) and subsequent skin marking, or better, under real-time US guidance [25, 141].
In screening for nonpalpable subcutaneous metastases of melanoma and cancers of the lung, breast, colorectum, stomach, or ovary and for melanoma recurrences, 7.5 MHz linear array transducers can dynamically screen a wide area of the body [1, 38], but this procedure will probably be replaced by FDG-PET scan as a whole-body staging procedure with an overall sensitivity and specificity of 89 and 96 % [102, 114]. CT is obsolete as it underestimates the number of lesions [101].
Ultrahigh resolution US (20–30 MHz) is being used in imaging of nodular and infiltrative epidermal and dermal lesions. The width of the field of view is 1.2 cm, and the depth of penetration only 1–2 cm.
US using frequencies of 15 MHz or more can clearly define the skin layer morphology including changes in the epidermal thickness [124]. Although epidermal lesions are visible, accurate clinical assessment of their depth of extension is not possible without ultrahigh-frequency (> = 20 mHz) US. Ultrahigh-frequency US is a sensitive tool in lesion detection and delineation of the deep margin of skin lesions. In the majority of lesions, however, it cannot differentiate malignant from benign lesions and will not obviate further need for biopsy [39]. Even with these ultrahigh-frequency transducers, the normal epidermis cannot be visualized. Exceptions are the sole of the foot and the hypothenar area. Hypodermis can be visualized as a hyperechoic layer.
In the detection of local recurrences of soft tissue sarcoma, MRI and US appear to be equally sensitive. US, however, is the most cost-effective method in the detection of early local recurrences of soft tissue sarcomas and should therefore be used for initial routine follow-up and guided biopsies [4]. The presence of a non-elongated, hypoechoic mass is highly suspicious for local recurrence [23]. However, US may be inconclusive in the early postoperative period (3–6 months postoperatively), as inhomogeneous, hypoechoic masses may also represent hematoma, abscess, or granulation tissue (Fig. 1.4). US follow-up with comparison to a baseline study on MRI, both performed 4–6 weeks after surgery, can help to differentiate such cases. US-guided FNAB and/or CNB represents a possible alternative. MRI diagnosis of a soft tissue tumor recurrence in the immediate postoperative period or after irradiation can be extremely difficult due to the diffuse high signal intensity background in (fast) spin-echo T2-weighted images or post-contrast (fat-suppressed) spin-echo T1-weighted sequences. If the tumor recurs in poorly vascularized postoperative scar tissue, intravenous gadolinium administration may have little effect in terms of tumor enhancement and thereby increased conspicuousness [23, 67]. If scar tissue and recurrence cannot be differentiated, US-guided or CT-guided percutaneous biopsy should be considered [11, 23]. Under those circumstances the exam has prime prognostic and therapeutic value [141].
Obtaining an MR time slot may be a practical problem in most institutions if the imaging-guided biopsy has to be performed on short notice. In a US- or CT-guided procedure, this problem does not exist. In addition, the technical staff is limited to US operator.
1.3 Superficial Soft Tissue Tumors and Tumorlike Lesions
Superficial soft tissue lesions are a distinct group of lesions in which US with CDUS has a more specific role improving the accuracy of the clinical diagnosis. Superficial soft tissue tumors can be classified into two categories: epidermal-dermal (or cutaneous) lesions and subcutaneous fat layer (or subcutaneous) lesions (Fig. 1.5a–c) (US of the normal skin and nail). The normal hyporeflective subcutaneous fat layer narrows with increasing external compression by the probe. Thickness of the dermis is variable, thin at the forearm and thick, as a result of high collagen content, in the lumbar region [138]. The subcutaneous fat layer is composed of hyposonant fat lobules interspersed with reflective fibrous septa (Fig. 1.5a–c). Cutaneous lesions include tumors originating from the skin appendages such as sweat glands, sebaceous glands, and hair follicles. Subcutaneous tumors include most mesenchymal tumors, but mesenchymal tumors can also occur in the dermal layer [8, 138].
Both cutaneous and subcutaneous tumors are mainly located in the subcutaneous fat layer rather than the dermis because the dermal layer is relatively strong and elastic compared with the subcutaneous fat layer. Benign cutaneous tumors include eccrine spiradenoma, chondroid syringoma, pilomatricoma, dermatofibroma, stump neuroma, schwannoma, and neurofibroma. Other benign tumors that may be located in the subcutaneous fat include vascular malformation, vascular leiomyoma, neurofibroma, schwannoma, lipoma, glomus tumor, and stump neuroma. Malignant cutaneous tumors include melanoma, metastasis, and lymphoma. Other malignant tumors that may be located in the subcutaneous fat are synovial sarcoma, lymphoma, and metastasis. Tumorlike lesions at the subcutaneous area consist of ganglion cysts, Langerhans histiocytosis, nodular fasciitis, rheumatoid nodule, fat necrosis, abscess, tumoral calcinosis, and epidermal inclusion cyst [51]. High-frequency US technique has been a valuable addition to the diagnosis. It allows a real-time view of the skin with discrimination of structures that measure ≥0.1 mm and provide highly useful information on the blood flow in the skin and its surroundings [138] (Table 1.1).
Table 1.1
Most frequent mass lesions with frequency and relative frequency at the skin and subcutaneous tissue in the series of Wortsman [138]
Group | Frequency (%) | Specific diagnosis | Relative frequency (%) |
|---|---|---|---|
Benign nonvascular tumors | 46 | Lymph node (enlarged) | 31 |
Lipoma | 28 | ||
Epidermoid cyst | 23 | ||
Pilomatricoma | 6 | ||
Articular and periarticular lesions | 17.5 | Synovial ganglion cyst | 60 |
Bursitis | 21 | ||
Synovitis | 19 | ||
Inflammatory and infectious lesions | 15.5 | Fat necrosis | 51 |
Fluid collections | 19 | ||
Fistulae | 8 | ||
Warts | 8 | ||
Hidradenitis suppurativa | 6 | ||
Benign vascular tumors | 9.2 | Hemangioma (hamartoma) | 65 |
Vascular malformation | 34 | ||
Nail lesions | 5.5 | Psoriasis | 68 |
Glomus tumor | 12 | ||
Granuloma | 9 | ||
Subungual exostosis | 4 | ||
Exogenous skin components | 3.5 | Cosmetic cutaneous fillers | 56 |
Foreign bodies (glass, wood, thorns) | 44 | ||
Malignant tumor | 1.7 | Basal cell carcinoma | 55 |
Squamous cell carcinoma | 24 | ||
Cutaneous metastasis | 8 | ||
Vascular non-tumoral lesions | 1.1 | Mondor disease (sclerosing thrombophlebitis of subcutaneous vessels) | 35 |
Nonsclerosing vascular thrombosis | 25 | ||
Anatomic variants | 40 |
1.4 US Findings in Specific Soft Tissue Tumors and Tumorlike Lesions of the Extremities
1.4.1 Articular and Synovial Sheath Masses
1.4.1.1 Synovial Osteochondromatosis or Synovial Chondromatosis
The condition known as synovial osteochondromatosis or synovial chondromatosis is a metaplastic transformation of synovial cells into cartilage. These cartilaginous nodules often calcify and/or ossify as nodules of equal size. US is the imaging modality of choice when the disease is suggested by clinical examination or radiographs [24]. Both exclusively cartilaginous and calcified nodules can be identified by US (Figs. 1.6 and 1.7). Due to its dynamic scanning ability, US can, in joints that are in the range of US, also differentiate freely moving bodies from nodules embedded in the synovium. Nodules may form an acoustic shadow front if calcified [91, 97, 106]. Synovial osteochondromatosis or chondromatosis usually presents as a monoarticular disease. Rarely bursae and tendon sheaths undergo synovial metaplasia.



Fig. 1.6
Synovial osteochondromatosis of the left shoulder joint in a 20-year-old woman. Transverse US image of the posterior, caudal aspect of the left shoulder (5 MHz curvilinear transducer). Marked distention of axillary recess (outlined by small arrows), filled with synovial proliferation. Embedded are multiple, equal-sized cartilaginous bodies. Synovial proliferation was also noted in the infraspinatus recess and biceps tendon sheath (not shown). The biceps tendon sheath contained multiple, partially calcified, metaplastic nodules. The patient was treated by synovectomy

Fig. 1.7
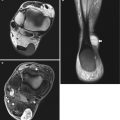
(a, b) Synovial osteochondromatosis of right hip joint in a 40-year-old white man with a 2 year history of right hip pain. (a) Axial CT scan section at tip of greater trochanter; bone window setting. (b) Longitudinal, anterior US image. One, larger, peripherally calcified nodule and numerous small, faintly calcified nodules can be identified, predominantly in the medial and anterior joint space (a) Marked distention of the anterior hip joint space (b) (between calipers). Intra-articular synovial proliferation; two embedded metaplastic nodules. The most proximal, calcified nodule demonstrates posterior acoustic shadowing
1.4.1.2 Tenosynovial Giant Cell Tumor (Pigmented Villonodular Synovitis (PVNS))
Pigmented villonodular synovitis (PVNS), also known as giant cell tumor when it affects the tendon sheath, is a benign inflammatory disorder resulting in diffuse or localized synovial hypertrophy.
In articular PVNS, US depicts hypoechoic synovial proliferation of variable thickness, affecting the entire synovial cavity or only a limited portion (Figs. 1.8 and 1.9). Lobulated soft tissue nodules may project from the synovium into a hypoechoic or anechoic joint effusion, as a result of debris or hemorrhage. Loculations of joint fluid may be created by the synovial infolding [70].



Fig. 1.8
(a, b) Pigmented villonodular synovitis (PVNS) involving the left talocalcaneonavicular joint in a 47-year-old man. (a) Lateral radiograph. (b) Longitudinal US image of dorsum of hind foot. (c) Sagittal gradient-echo T2-weighted MR image. Radiograph shows dense soft tissue mass along the dorsal aspect of talus and navicular which contains a single calcification. (a) Well-defined, inhomogeneous, but predominantly hypoechoic solid soft tissue mass. Minute anechoic foci and small hyperechoic areas are also present. (b) Note secondary pressure erosion of the talar neck. The low signal intensity hemosiderin-laden soft tissue mass involves the talonavicular and communicating anterior and middle subtalar joint (c)

Fig. 1.9
(a, b) Bifocal pigmented villonodular synovitis of the right knee. (a) Sagittal gradient-echo MR image. (b) Longitudinal US image; split screen comparison view of dorsal femorotibial joint space. Marked distention of posterior femorotibial joint space, filled with soft tissue mass of intermediate signal intensity. (a) Multiple foci of low signal intensity are present both in the deep and superficial dorsal aspect of the mass. Intraosseous tumor extension in the dorsolateral aspect of the tibial proximal metaphysis (a, arrows) is noted as well. The symptomatic right side (b) shows a slightly inhomogeneous, predominantly hypoechoic synovial soft tissue mass, enveloping the posterior cruciate ligament insertion. The mass displaces the posterior capsule. A second tumor focus was localized in the medial aspect of the suprapatellar pouch and was biopsied
Rheumatoid arthritis, seronegative inflammatory arthritis, hemophilic arthropathy, and gout arthritis should be considered in the differential diagnosis of diffuse synovial hypertrophy on US [70].
PVNS of the tendon sheath is called giant cell tumor and is a common tumor at the hand.
US depicts it as a well-defined, occasionally slightly nonhomogeneous or lobular, hypoechoic, solid soft tissue mass, abutting or eccentrically enveloping the tendon [4, 11, 60, 72] (Fig. 1.10).


Fig. 1.10
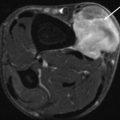
Giant cell tumor of tendon sheath at the laterovolar aspect of the proximal phalanx of the middle finger in a 44-year-old male patient. (a) Longitudinal US view demonstrating oval mass lesion at the subcutaneous tissue, lateral adjacent to the flexor tendons, superficial part is hyporeflective, deeper part is more reflective. (b) Longitudinal CDUS demonstrating marked vascular signal at the superficial hyporeflective part
1.4.1.3 Amyloidosis
β2-Amyloid arthropathy occurs in patients undergoing long-standing hemodialysis (more than 5 years) with the cuprophane membranes and in patients with multiple myeloma. The cuprophane membranes’ related condition is prone to extinction as these membranes are no longer used. Amyloid arthropathy may involve every joint in a systemic way. Articular deposits are characterized by thickening of the synovium, and subchondral articular intraosseous amyloid deposits are demonstrated with increasing number and diameter over time and described as growing bone cysts [50]. The US parameters of shoulder amyloid arthropathy are enlargement of the rotator cuff tendons (supraspinatus tendon larger than 8 mm in thickness, its normal range being 4–8 mm), focal intratendinous areas of increased echogenicity, distention of the glenohumeral joint space, the synovial tendon sheath of the long head of the biceps and the subacromial-subdeltoid bursa, irregularity of the humeral head, and abnormal fluid collections around the joint [46] (Figs. 1.10 and 1.11).


Fig. 1.11
Symmetrical amyloid shoulder arthropathy in multiple myeloma patient. Transverse US image of the anterior aspect of the shoulder. Marked distention of the subacromial-subdeltoid bursa (arrows) and the synovial tendon sheath of the long head of the biceps, causing anterior displacement of the transverse ligament (arrowheads). Mixed predominantly hypoechoic and faintly hyperechoic synovial amyloid deposits
The capsular and articular or bursal synovial amyloid deposits have a slightly heterogeneous hypoechoic echotexture.
1.4.2 Peripheral Neurogenic Tumors
Large peripheral nerves of the extremities, such as the sciatic, popliteal, ulnar, and median nerves, can be routinely identified by high- and ultrahigh-resolution real-time US [16, 36, 52, 60, 80]. In a high-frequency US examination, also smaller superficial peripheral normal nerves can be identified as a hyperechoic, fascicular soft tissue structure in its course between muscle bellies and at the subcutaneous tissue (sural nerve) [22]. The configuration is concentric or oval in transverse section and tubular on longitudinal view. The nerve roots of the brachial plexus are homogeneous hyporeflective, whereas the peripheral nerves show on ultrahigh-frequency transducers an alternating pattern of hypo- and hyperechogenicity. The parallel-oriented, but discontinuous, linear, hypoechoic areas represent coalescing bundles of neuronal fascicles, embedded in a hyperechoic background of connective tissue, called endo-, peri-, and epineurium. US underestimates the number of neuronal fascicles, when compared with histological sections. Presumed explanations are the undulating neural course and its resultant obliquity and lateral deformation. With the use of lower US frequencies, the hypoechoic areas within the nerve become less defined and less numerous as a result of degradation in image resolution [80].
The nerve remains immobile in comparison to its surrounding musculotendinous structures during (passive or active) dynamic examination. This is best visualized on longitudinal view. A slight nerve translation on longitudinal view is depicted at the level of the forearm nerves while the head is bended to the contralateral side. Normal nerves are flattened out by transducer compression in transverse view.
Of key importance in the diagnosis of a peripheral neurogenic tumor is the recognition of the location along the peripheral nerve course.
1.4.2.1 Peripheral Nerve Sheath Tumors
Tumors of peripheral nerves are rare, usually benign, and subcutaneous in location. US reports have documented schwannoma, neurofibroma, and neural fibrolipoma (formerly called fibrolipohamartoma) [9, 11, 21, 29, 59, 62, 64, 77, 78] (Fig. 1.12). In the series of Hung et al., nerve sheath tumors were the third most frequent diagnosis superficial to the investing fascia (16/247) with a US diagnostic sensitivity and specificity of 68.8 and 95.2 %, respectively [1]. With the exception of intraneural ganglion and neural fibrolipoma, all these nerve-related tumors and tumorlike lesions were hypoechoic masses [16, 21, 29, 35, 59, 60, 62, 77, 78]. A plexiform neurofibroma was reported as an almost echo-free mass with poor back wall enhancement [105] (Fig. 1.13). The majority of reported schwannomas and two neurofibromas, both of them in von Recklinghausen’s disease, showed posterior acoustic enhancement [16, 21, 62] (Fig. 1.14). A tarsal malignant peripheral nerve sheath tumor (MPNST), arising at the site of multiple, postsurgical in situ recurrences of an initial schwannoma, showed poor delineation, homogeneous hypoechogenicity, and some dorsal acoustic enhancement.




Fig. 1.12
(a–c) Long-standing fibrolipohamartoma of median nerve (MN) in a 54-year-old woman. (a) Sagittal spin-echo T1-weighted MR image of the wrist. (b) Axial SE T1-weighted MR image proximal to the carpal tunnel. (c) Transverse US image of carpal tunnel. Enlargement of the median nerve in the distal forearm (b), carpal tunnel (c), and metacarpus. (a) The enlarged median nerve contains dot-like thickened neuronal fascicles and some fatty tissue, especially in its deep aspect (b and c). The thickened bundles of neuronal fascicles are of intermediate signal intensity in (a and b) and hypoechoic in (c)

Fig. 1.13
Schwannoma (ancient) of the right median nerve at the forearm in a 45-year-old male patient. Painless firm mass lesion at the flexor side of the forearm. (a) Longitudinal US with CDUS demonstrates an oval mass lesion with nonhomogeneous hyporeflective aspect and sharp margins along the course of the median nerve. The median nerve is clearly demonstrated entering and leaving the lesion. Maximal diameter of the lesion is 4 cm. CDUS shows multiple intralesional vascularization with nonhomogeneous distribution. (b) Coronal SE T1-WI MRI after IV gadolinium administration reveals nonhomogeneous enhancement of the lesion

Fig. 1.14
(a, b) Schwannoma of median nerve in forearm. (a) Longitudinal linear 5 MHz US image. (b) Longitudinal linear 7.5 MHz US image. The median nerve (MN) courses in and out of the well-defined hypoechoic nerve sheath tumor (a) The internal echotexture of the tumor drastically changes when the 7.5 MHz transducer is applied (b)
1.4.2.2 Nerve-Related Tumorlike Lesions
Intraneural ganglion is a cystic, glue-like mass containing fluid and lined with collagen within the epineurium that may cause pain and motor dysfunction due to compression [77, 78]. These are nonneoplastic cysts caused by the accumulation of thick mucinous fluid through a connecting branch with a neighboring joint with growth within the epineurium of peripheral nerves [123]. Histological examination shows nerve fibers dispersed within the mucinous substance of the cyst.
Intraneural ganglia are well known at periarticular sites. Most frequently the peroneal nerve is involved, with drop foot at presentation. US shows a spindle-shaped anechoic soft tissue structure within or abutting the nerve course [77, 78].
These ganglia are common along the course of the suprascapular nerve in the shoulder [57, 120, 126, 127]. They invariably cause infraspinatus weakness and in some cases supraspinatus weakness as well.
Other locations are the tibial nerve, ulnar nerve, sural nerve, and sciatic nerve [99]. US-guided aspiration and injection of corticosteroid of intraneural ganglia is recently described as an alternative to surgical decompression that is the standard of care [83]. In the last 4000 shoulder US studies we conducted, we recognized this entity in five cases. Two of them were cured by repetitive aspiration under US guidance.
In a reported tuberculoid leprosy of the external popliteal nerve [35], the well-defined hypoechoic mass proved surgically to be a caseous pouch. Within it, the thickened sheath of the enlarged lateral popliteal nerve could be identified as two parallel linear hyperreflectivities on longitudinal view.
Traumatic neuromas occur in postsurgical, postamputation, or posttraumatic patients [11, 16, 64]. Traumatic friction or irritation of a nondisrupted nerve trunk as well as partial or complete transection of the nerve may induce this failed repair mechanism [119]. A traumatic neuroma usually presents as an ill-defined, hypoechoic mass. These neuromas in traumatic complete neurotmesis or amputation are located at the proximal end of the cutted nerve and are called end-neuroma (Fig. 1.15). Sidebulb neuromas are typically formed in incomplete nerve lesion or axonotmesis.


Fig. 1.15
Amputation neuroma at the distal stump of the left sciatic nerve in a 35-year-old male patient, amputation above the knee related to trauma. (a) Sagittal US image at the posterior aspect of the thigh demonstrates a hyporeflective oval mass at the end of the sciatic nerve (arrows). (b) Sagittal TSE T1-WI FS image after intravenous gadolinium contrast administration, partially enhancing mass at the end of the tortuous sciatic nerve (arrows)
Morton’s neuroma is no real neuroma but represents focal perineural fibrosis involving a plantar digital nerve [82, 103, 104]. It occurs in between the metatarsal heads and is quite common. Most commonly affected is the digital nerve of the third web space, followed in decreasing order of frequency by web spaces two, one, and four. Neuroma can be solitary or can simultaneously involve multiple web spaces. Bilateral lesions may also occur. The neuroma is at least 5 mm in size in the majority of cases (95 %). If greater than 20 mm in length, the interdigital mass is suspicious for an abnormality other than neuroma, such as a ganglion cyst, a synovial cyst, or a giant cell tumor (GCT) from an adjacent tendon sheath [63].
Middle-aged women are most commonly affected, and they typically complain of pain and numbness in the forefoot, elicited by ambulation and mediolateral compression of the forefoot, when narrow-toed shoes are worn. The normal plantar nerve is only sonographically detectable with high-resolution US. In the presence of a neuroma, US can identify on longitudinal views the plantar digital nerve coursing into the pseudotumoral mass. The abnormal, possibly edematous, nerve is linear, 2–3 mm thick, and hypoechoic; its demonstration in continuity with the interdigital mass improves diagnostic confidence. Morton’s neuroma is a predominantly well-defined but occasionally poorly defined soft tissue structure. Majority are hypoechoic masses, and minority demonstrate a mixed echopattern or anechogenicity (Fig. 1.16). They present poorly or not vascularized on power color Doppler. A plantar transducer approach is preferred with imaging in both the longitudinal and transverse plane. The correct transverse section should visualize the hypoechoic rim of cartilage covering the corresponding metatarsal heads.


Fig. 1.16
Morton’s neuroma located at the second (left image) and third (right image) web space of the left foot in a 60-year-old female patient. US examination with plantar approach and transverse imaging plane during lateral compression of the metatarsal heads demonstrating a small hyposonant lesions. While compressing the metatarsal heads, the lesion jumps to the plantar aspect of the foot, diameter 7 mm (right image) and 6 mm (left image)
Extreme flexion of the toes in the direction opposite the transducer or the Mulder maneuver (mediolateral compression of the forefoot and manual digital plantar displacement of the soft tissues in the examined web space with the transducer applied to the sole of the foot) help in rendering the neuroma more superficial and allow it to be better appreciated [63, 82].
In case of major callus at the foot sole, longitudinal dorsal interdigital view may demonstrate the neuroma. In this approach plantar active pressure between the metatarsal heads to decrease the distance between the lesion and the probe is needed.
1.4.3 Pericytic (Perivascular) Tumors
1.4.3.1 Glomus Tumor
Glomus tumors (GTs) are small but extremely painful skin tumors of mesenchymal origin. Glomus tumors originate from the neuromyoarterial glomus bodies and have a homogeneous, markedly hypoechoic, or even sonolucent echotexture (Fig. 1.17). Predilection site is the fingertip, although the tumor may occur everywhere. In the distal finger, the subungual space is more affected than the pulpar soft tissues [4].


Fig. 1.17
(a, b) Glomus tumor of the distal phalanx of digit 3. (a) Longitudinal US image. (b) Sagittal gradient-echo MR image. US identifies the 5 mm hypoechoic nodule (a, between calipers) along the palmar aspect of the tuft (FT). MRI depicts intermediate signal intensity tumor (b) causing pressure erosion of the underlying cortex
Average lesion size is 6 mm, and lesions as small as 2 mm can be detected.
Therefore, US investigation with at least a linear array 10 MHz transducer is recommended. Although exquisitely tender to palpation, most lesions are not palpable as such.
Glomus tumor may have a flattened configuration when subungually localized and in that case may present as a less conspicuous, thickened hypoechoic subungual space. The normal subungual space is only 1–2 mm thick. If localized lateral to the nail bed or in the palmar digital soft tissues, it assumes an ellipsoid or concentric shape [37, 41]. These lesions show marked (power) Doppler vascular signal that equals vascular signal of the normal nail bed (Fig. 1.18). Differential diagnosis of a thickened hypoechoic subungual space should include angioma and mucoid cyst. Epidermoid inclusion cysts can also present as small, concentric, hypoechoic solid soft tissue mass underlying the nail matrix but show no marked Doppler activity [4, 43].


Fig. 1.18




Subungual glomus tumor at the left annular finger in a 50-year-old female patient. US (right longitudinal and left transverse views) demonstrates small mass lesion with marked CDUS activity. Thickening of the distal subungual tissue with maximal diameter 1.3 cm, elevation of the nail and erosion of the distal phalanx
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree