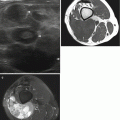
Fig. 21.1
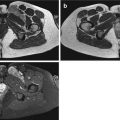
Accessory palmaris longus muscle in a 15-year-old boy. Axial T1-weighted MR image after gadolinium contrast injection. The MR image reveals an additional mass, located superficially of the flexor digitorum tendons, with similar MR characteristics as normal skeletal muscle
In the lower extremities, anatomic variants occur almost exclusively in the soleus muscle [52]. Though present from birth, an accessory soleus muscle usually manifests in the late adolescent age because of muscle hypertrophy secondary to increased physical activity, especially in athletes [139]. It arises either from the anterior surface of the soleus muscle or from the soleal line of the tibia and fibula and appears as a soft tissue mass between the medial malleolus and the Achilles tendon [52, 139]. Up to 25 % of patients may present with an asymptomatic soft tissue swelling medial to the os calcaneus [139] (Fig. 21.2). Symptoms, when present, have been attributed to closed compartment ischemia and are accentuated by exercise [52].
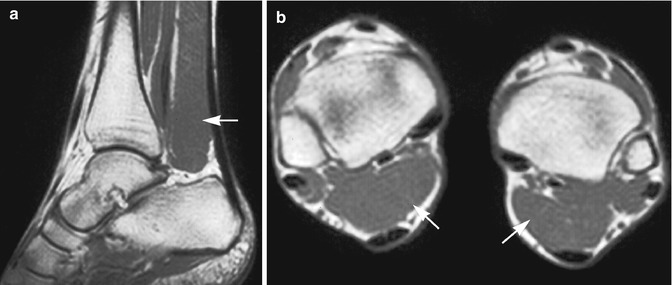

Fig. 21.2
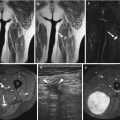
Accessory soleus muscle in an adult man: (a) sagittal spin echo T1-weighted MR image; (b) axial spin echo T1-weighted MR image. There is a muscle belly within Kager’s fat triangle, anterior to the Achilles tendon (arrows). Signal intensity and bilateral presentation of abnormality are in favor of accessory soleus muscle
Herniation of muscle through fascial planes can also mimic a tumor. It can be found in athletes, soldiers, or other professions requiring great strains on the legs. Most of the herniations have a constitutional origin, where muscle strain or hypertrophy leads to rupture of fascia on specific constitutional weaker locations [118]. The anterior tibial compartment is a common location for muscle herniations, where the herniation is palpable as a soft tissue mass [85]. Herniation of the m. extensor digitorum longus, m. peroneus longus and brevis, and m. gastrocnemius is also possible [118]. This can be asymptomatic, but also more prominent after exercise [81, 118]. Herniations can be multiple and bilateral [27] (Fig. 21.3).
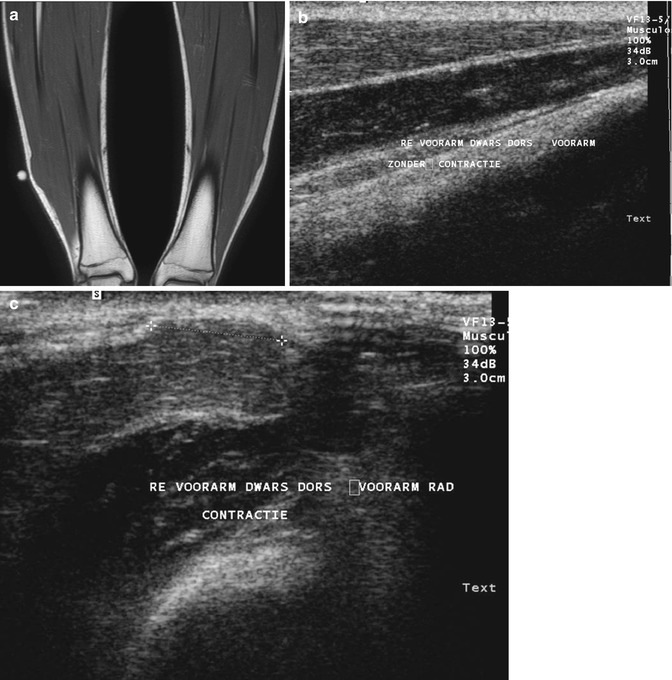

Fig. 21.3
Muscle herniation in two different patients. (a) Long-standing bilateral muscle herniation in a 15-year-old male: a coronal spin echo T1-weighted MR image. A focal bulging of the peroneus muscle compartment is seen, more pronounced on the right side. As expected, this protruding mass has the same signal characteristics of normal muscle tissue (b, c). Ultrasound of the forearm without (b) and with muscle contraction (c) in another patient. Note the presence of a mushroom-shaped lesion bulging through a focal defect in the superficial fascia. The lesion is only visible with muscle contraction (c)
The diagnosis of a muscular anomaly is mainly based on knowledge of the most common locations and the aspect of the lesion on imaging. Both anatomy variations and muscle herniations can be depicted with ultrasound, where the suspicious mass is identified as having the same ultrasound appearance as normal muscular tissue. The ability of dynamic evaluation further increases the diagnostic accuracy. As expected, the signal characteristics on MR imaging of these lesions are identical to skeletal muscle on all pulse sequences, as long as there is no adjacent edema or contusion. When in doubt, a dynamic MR examination with forced dorsiflexion and plantar flexion of the ankle allows a better evaluation of the changes in shape and size of a muscle herniation [27, 118]. MR imaging of the fascial defect is possible but difficult.
Other anomalies such as an accessory breast or nipple may mimic a soft tissue tumor (Fig. 21.4). It is usually present along the primitive milk line above or below the normal breast location and is the most frequently encountered congenital anomaly of the breast [103]. Other more rare locations include the axilla, scapula, thigh, and vulva [102], since the primitive milk line extends from the axilla to the groin. Patients with accessory breast tissue may be asymptomatic or may present with swelling and pain. As accessory breast tissue is subject to the same spectrum of physiological changes and pathological processes as proper breast tissue it may therefore come to attention during menarche, pregnancy, lactation, or in case of benign and malignant breast disease [46, 67].
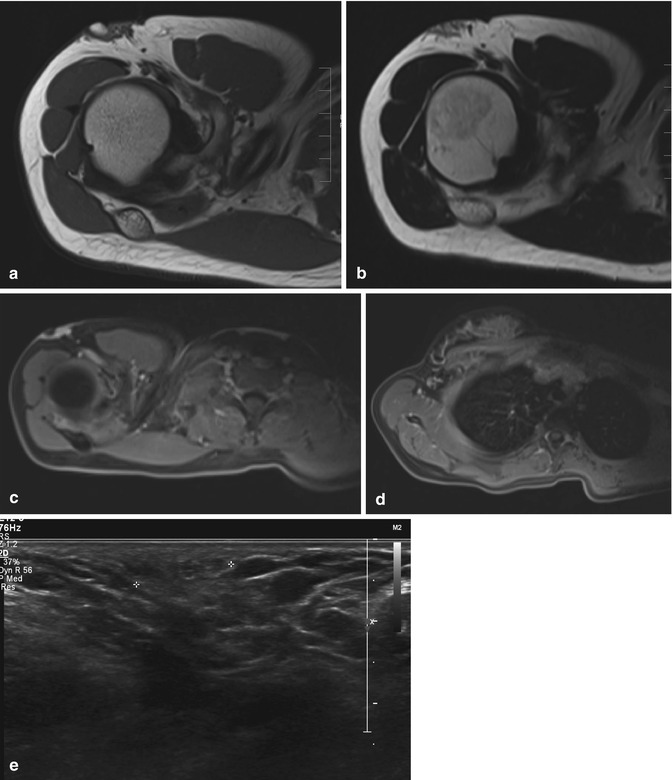

Fig. 21.4
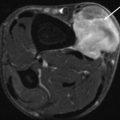
Accessory breast in a 21-year-old female with a palpable swelling in the right axilla. The lesions enlarge and are slightly painful in the second half of the menstrual cycle: (a) axial T1-weighted MR image; (b) axial T2-weighted MR image. (c, d) Axial FS T1-WI MR image before (c) and after intravenous injection of gadolinium contrast (d). Presence of a small soft tissue “mass” ventrolateral in the right axilla on both pulse sequences, with signal intensities comparable to the signal intensity of normal adjacent breast. The lesion enhances similarly as breast tissue (d) Ultrasound (e) shows an echotexture similar as breast tissue
21.2.2 Inflammatory and Infectious Lesions
21.2.2.1 Cellulitis
Acute infectious cellulitis is an infection of the subcutaneous fat not extending beyond the superficial fascial planes (Fig. 21.5). It is usually associated with a hemolytic group A Streptococcus infection. Cellulitis will only on rare occasions present as a soft tissue mass [174]. An association with abscesses, ascending lymphangitis, regional lymphadenitis, osteomyelitis, and pyoarthrosis is possible [79]. Lymphedema may mimic infectious cellulitis; but the latter is more localized than lymphedema, which tends to affect the entire extremity.


Fig. 21.5
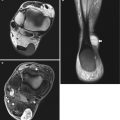
A 24-year-old woman with an infection of the lower leg. Axial turbo spin echo T2-weighted MR image. Thickening of the skin and subcutaneous fat, with multiple septations of high signal intensity, corresponding to edema/cellulitis. The gastrocnemius muscle also has an abnormal signal intensity and appears atrophic
CT shows diffuse infiltration of the subcutaneous fat and thickening of the skin. On MRI, cellulitis appears as an ill-defined area, hypointense on T1-weighted sequences and hyperintense on T2-weighted sequences [150]. Cellulitis may be diagnosed when T2-weighted images reveal subcutaneous thickening with fluid collections and when subcutaneous tissue, superficial fascia, or both show contrast enhancement [165]. The depth of soft tissue involvement of the infection can be best evaluated on T2-weighted images [150]. However, as the sensitivity of magnetic resonance imaging exceeds the specificity, the extent of the deep fascial involvement can be overestimated [165], leading to the wrong diagnosis of necrotizing fasciitis.
21.2.2.2 Necrotizing Fasciitis
Necrotizing fasciitis is a rare soft tissue infection, involving deep fascial planes. It has a predilection for older patients, especially for those with malignancy, poor nutrition, and alcohol or drug abuse. It can also be found after trauma, or around foreign bodies in surgical wounds. However, it is important to remember that it can also appear in otherwise healthy subjects with no known risk factors. Early recognition is critical, since this entity is a surgical emergency. The clinical course can be fulminant and the mortality rate can be as high as 73 % [150]. The causative organisms are mostly group A hemolytic Streptococcus and Staphylococcus aureus, on occasion acting in synergy. Other both aerobic and anaerobic pathogens may also be involved [34].
Necrotizing fasciitis has similar signal behavior on MR as cellulitis, except for a deeper extension. A hyperintense signal on T2-weighted images in deep fasciae with fluid collections, thickening, and peripheral enhancement after intravenous contrast medium injection suggests necrotizing fasciitis [165]. However, this is not typical for necrotizing soft tissue infection, as other non-necrotizing conditions can have similar MR signal characteristics [112]. Therefore, clinical correlation is of utmost importance [34].
21.2.2.3 Lymphedema and Lymphangitis
Lymphedema is classified as primary or secondary lymphedema. The primary form is more common in children and is associated with a variety of hereditary or genetic syndromes [194]. Secondary lymphedema has no age preference. Local causes include trauma, surgery, infection, chronic inflammation, and radiotherapy (Fig. 21.6). It can also be associated with systemic disease, with a more generalized edema. Secondary lymphedema is usually a clinical diagnosis.


Fig. 21.6
Lymphedema of the left upper leg in a 48-year-old woman, with a history of vulval carcinoma treated with surgery and radio- and chemotherapy. Axial turbo spin echo T2-weighted MR image. There is an important thickening of the subcutaneous fat secondary to lymphedema, probably as a consequence of the intrapelvic surgery. Note the important increase in volume of the left thigh compared with the contralateral leg. An inflammation of the left adductor muscles can also be seen
MRI of chronic lymphedema reveals deformity of lymphatics at different tissue levels [107]. In the subcutaneous tissue, it shows a diffuse edema or a honeycomb pattern consistent with reticular lymphangiectasia and “lakes” with increased signal intensity on T2-weighted images [107].
21.2.2.4 Abscess
A soft tissue abscess is a well-delineated fluid collection surrounded by a well-vascularized fibrous pseudocapsule. It can present as a soft tissue mass without a suggestive history or symptoms [85], and the radiologist must therefore always consider a possible infectious origin of a mass with undetermined characteristics. In one-third of cases, abscesses are multiple [85]. Associated inflammation is possible, distorting normal muscle anatomy and fascial planes [16]. Depending on the causal organism and degree of inflammation, the margins of an abscess can be well-defined or infiltrating.
Conventional radiography has little value, unless there is gas development within the abscess (Fig. 21.7). Ultrasound shows an elongated or lobulated fluid collection, which may show peripheral vascularity on color Doppler (Fig. 21.8). In case of intralesional gas, dirty shadowing may be present (Fig. 21.9). It is also useful in guiding an aspiration biopsy or percutaneous catheter drainage.




Fig. 21.7
Gas gangrene of the upper arm in a young man. Plain radiograph. Presence of a soft tissue swelling with intralesional air collection and an air-fluid level, indicating soft tissue abscess

Fig. 21.8
Soft tissue abscess. Ultrasound shows a hypoechogenic collection with peripheral color Doppler signal

Fig. 21.9
Intramuscular abscess. Ultrasound shows a hypoechogenic collection intralesional dirty shadowing in keeping with gas bubbles
On MR imaging, an abscess is hypointense to isointense relative to muscle tissue on T1-weighted images. On T2-weighted images, the central portion of the abscess is usually hyperintense, but the capsule may display an isointense or hypointense signal intensity relative to subcutaneous fat [85]. On T1-weighted images, the pseudocapsule can have a variable signal intensity compared to skeletal muscle. After intravenous contrast medium injection, a peripheral rim of enhancement is seen, corresponding to the inflammatory and cellular component of the abscess (Fig. 21.10). Enhancement after intravenous contrast medium injection can also be absent [85].


Fig. 21.10
A 65-year-old man with untreated diabetes mellitus: (a) axial proton density-weighted MR image with fat suppression; (b) axial spin echo T1-weighted MR image after gadolinium contrast administration. Besides a diffuse cellulitis and lymphedema, the images also reveal a fluid collection, located in the lower leg between the extensor digitorum and tibialis anterior muscles, with spontaneous high signal intensity on the proton density images (a). After contrast administration a peripheral enhancement can be seen, corresponding to granulation tissue at the periphery of a soft tissue abscess (b)
A variable degree of peripheral edema in muscle and subcutaneous tissue can be seen, displaying a hyperintense signal intensity on T2-weighted images. Inhomogeneity on T2-weighted sequences may be a consequence of intralesional gas bubbles and/or necrotic material [190].
The described signal characteristics can be different in an immunocompromised host [85]. The peripheral edema usually seen on T2-weighted images is sometimes absent. Similarly, T1-weighted images will not always show the pseudocapsule. The infected fluid in the center of the abscess can have an inhomogeneous signal intensity [9]. If the content is sufficiently viscous, it can even show mild increased signal intensity on T1-weighted images [21]. In the proximity of bone, a soft tissue abscess is often associated with osteomyelitis or a periosteal reaction (Fig. 21.11).


Fig. 21.11
(a, b) Sacral osteomyelitis and multiple pelvic abscesses. (a) Oblique FS coronal T2-weighted MR image; (b) oblique coronal FS T1-WI MR image. (b) Oblique coronal contrast-enhanced FS T1-WI MR image. (c) Axial contrast-enhanced FS T1-WI MR image. Bilateral T2-hyperintense presacral collections (a) with peripheral rim enhancement (b, c). Note increased T2-signal and contrast enhancement of the sacrum in keeping with sacral osteomyelitis
21.2.2.5 Pyomyositis
Pyomyositis, also called bacterial myositis, is a rare cause of single or multiple abscesses of skeletal muscle of unknown etiology. It was initially mainly found in tropical regions where lack of footwear, insect bites, and minor trauma, if untreated, may lead to pyomyositis. Diabetes, HIV infection, and malignancy can, as immune-compromising conditions, however, also predispose to pyomyositis [35, 90, 144, 147], as such contributing to an increased incidence of this disease in industrialized regions with a more temperate climate. It is considered one of the most common musculoskeletal complications of AIDS [144, 154]. In 70–90 % of cases, the infection is caused by Staphylococcus aureus [35, 90, 144, 147]. Other pathogens such as Streptococcus pyogenes, Mycobacterium tuberculosis, Mycobacterium avium-intracellulare, Nocardia asteroides, Cryptococcus neoformans, and Toxoplasma, Salmonella, and Microsporidia species have also been reported [195].
In general, normal skeletal muscle has a high intrinsic resistance to bacterial infection and abscess formation. Therefore, some authors suggest that underlying muscle damage may facilitate the onset of pyomyositis. This is supported by the presence of previous trauma to the affected muscles in 20–50 % of cases [35, 72].
The clinical course of disease can be divided into three stages [39]. The first stage or invasive phase occurs during the first 2 or 3 weeks after infection, with subacute presentation such as local swelling, mild pain, variable fever, and anorexia. Diagnosis in this stage is difficult due to multiple differential diagnoses. During the second or suppurative phase, definitive abscess and pus are clearly evident. High fever, chills, local tenderness, swelling, and myalgia are frequent findings. It should be noted that local erythema and regional adenitis are usually absent in pyomyositis [45]. Aspiration of the lesion at this time reveals pus. The third stage of the disease is so severe that even toxic shock syndrome has been reported [83].
However, symptoms may be absent when the lesion is deep-seated or due to a superimposed transient bacteremia [144].
Laboratory data are not specific for pyomyositis. Leukocytosis and an increase in the erythrocyte sedimentation rate are often seen, but may be absent in patients with neutropenia or end-stage acquired immunodeficiency syndrome. Eosinophilia is reported only in tropical cases and may be due to a parasitic cause of infection [44, 45]. Interestingly, creatine phosphokinase and aldolase levels remain normal. Blood cultures are positive in only 5–30 % of patients and in 1.8 % the outcome is fatal due to sepsis and shock [110, 195]. Culture of aspirated pus has been reported as negative in 15–30 % of cases [35, 44].
The muscles of the thigh and gluteus region are most often affected. Pyomyositis has also been described in the obturator, serratus anterior, deltoideus, triceps, biceps, iliopsoas, gastrocnemius, abdominal, and paraspinal muscles (Fig. 21.12) [35, 79, 90, 147]. In AIDS patients, pyomyositis may be multifocal 43 % of cases in the study of Fleckenstein et al. [58]. Multiplicity of lesions in AIDS patients is not specific for pyomyositis and may be found in other pathologic conditions such as polymyositis, Kaposi sarcoma, and lymphoma [58].


Fig. 21.12
Pyomyositis in 2-year-old child. Coronal T2-weighted MR image shows diffuse increased signal within the right upper arm muscles
Magnetic resonance imaging is the most precise technique to use in determining the exact location and defining the extent of the disease [51], and it plays a pivotal role in early diagnosis. On T1-weighed images the abscess collection has a low signal intensity compared with surrounding muscle tissue. On occasion, a high-intensity peripheral rim is noted, probably representing blood breakdown products [72, 110]. Pus in the abscess can have an intermediate to high signal on T1-weighed images depending on the protein content. T2-weighed images reveal a hyperintense collection in the affected muscle, with increased signal in the surrounding muscle tissue representing edema, organized phlegmonous collections, or hyperemia [154]. Intravenous administration of contrast material can further discriminate between viable and necrotic muscle tissue, the latter lacking enhancement.
On occasion the imaging presentation of pyomyositis can be confused with a sarcomatous lesion, especially when further clinical and biochemical information is inconclusive. Key elements in the differential diagnosis favoring an infectious origin are the extension of the perilesional inflammatory reaction and the possible association of cellulitis. However, previous surgery or local radiotherapy at the site of a sarcoma may be accompanied by locoregional stranding, which may cause difficulties in the differential diagnosis [72].
Scintigraphy is rarely used for detection [202], but may detect additional abscesses on a distance of the primary lesion [147].
Pyomyositis can be effectively treated by antibiotic therapy in the first stage of disease, but during the later stages, drainage should be initiated early and serve as the main component of therapy, either by percutaneous computed tomography (CT) or ultrasound-guided drainage or by open surgery [72].
21.2.2.6 Muscular Cystic Echinococcosis
Human hydatid disease is a parasitic infection due to the development in the organism of Echinococcus granulosus larvae, a parasitic tapeworm, with dogs being the definitive hosts, sheep the intermediate hosts, and humans the incidental intermediate hosts. Ingestion of contaminated water or food and contact with dogs are known causes of infection. The disease is still endemic in several regions and has the highest incidence in the Mediterranean Basin, Middle East, South America, New Zealand, Australia, Central Asia, and China [138]. According to various authors, the incidence of musculoskeletal echinococcosis including involvement of subcutaneous tissue is around 1–5.4 % among all the cases of hydatid disease [10]. Alveolar echinococcosis due to infection by Echinococcus multilocularis is more rare, but has a more invasive nature sometimes mimicking a malignant lesion. Muscular echinococcosis may be primary, but may also occur secondarily resulting from the spread cysts from other areas either spontaneously or after operations for cystic echinococcosis in other regions of the body [87, 88]. The growth of cysts within a muscle is difficult because of muscle contractility and the presence of lactic acid. The affinity for muscles of the neck, trunk, and limb roots could be explained by the volume of the muscle mass, the increased vascularity, and the decreased activity of these muscle groups [15].
Primary muscular cystic echinococcosis disease is rare, with only isolated cases being described in the literature [15, 201]. Only few cases of primary subcutaneous cystic echinococcosis of the inferior limbs have been reported. Cystic echinococcosis of superficial muscles presents as a painless soft tissue mass with fluctuating consistency and evolves slowly. This mass may be accompanied with neurovascular compression signs, inflammation in case of fissuring, infection of the cyst [69], or skin fistula. Deep muscular cystic echinococcosis especially of the psoas muscle which is relatively more frequent [158] may be discovered by chance. Hydatid serology is often negative [122]. It is particularly useful in postoperative monitoring when looking for a local or more remote recurrence [62].
Muscular cystic echinococcosis may have different appearances on imaging techniques.
MR has proven superior over ultrasound in detecting this multivesicular structure. More solid appearances are also possible, making it sometimes difficult to differentiate it with other soft tissue tumors [107]. Even in these cases, MR can often reveal the vesicular nature of the lesion, which if frequently still focally preserved.
Ultrasonography and magnetic resonance imaging are the imaging tools of choice to confirm the diagnosis before surgery [62]. The imaging characteristics of soft tissue involvement resemble those of hydatid cysts found in the liver. Ultrasonographic appearance of cystic echinococcosis may vary. The cyst wall usually manifests as an echogenic line. Simple cysts (type I) do not demonstrate internal structures. Detachment of the endocyst from the pericyst (type II) appears as well-defined fluid collection with a localized split in the wall and “floating membranes” inside the cavity [17]. Multivesicular cysts (type III) manifest as well-defined fluid collections in a honeycomb pattern with multiple septa representing the wall of the daughter cysts (Fig. 21.13). Multivesicular or inhomogeneous muscle hydatids may have a nonspecific appearance that may simulate hematoma, abscess, or necrotic soft tissue tumor [62, 68]. Calcified cysts may be seen. Computed tomography is useful in doubtful cases, in deep muscular localizations, and in appraising the exact localization of the cyst and its relationship with the neural and vascular elements as well as the state of the adjacent bone. MRI imaging characteristics may differ depending on the life cycle stage of the parasite [162]. Typically, the lesion consists of a mother cyst, containing multiple daughter vesicles. On T1-weighted images these daughter vesicles are seen as hypointense cysts within the intermediate signal of the mother cyst. The signal intensity of the daughter cysts on T2-weighted images can be high or low, with some authors suggesting a relation with the presence and absence, respectively, of viable scolices [15]. A rim of low [15] signal intensity on T2-weighted images surrounds the lesion. This rim is composed of three layers: an endocyst, ectocyst, and adventitia. The adventitia develops as a reaction following compression and inflammation of surrounding tissue. It is well vascularized, enhancing after intravenous contrast injection [111, 120] (Figs. 21.14 and 21.15). Edema or acute inflammation caused by compression in soft tissue adjacent to the cyst is uncommon but may be seen.




Fig. 21.13
Soft tissue echinococcosis of the calf. Ultrasonography shows a well-defined multicystic fluid collection with vesicles, calcifications, and small echoes corresponding to hydatid sand

Fig. 21.14
Multicystic echinococcus cyst in the right paravertebral muscles: (a) axial FS T2-weighted MR image; (b) sagittal FS T2-weighted MR image; (c) axial T1-weighted MR image after gadolinium contrast injection. Large multicystic lesion is seen on T2-WI. (a, b) Because of vascularization of the pericyst, peripheral enhancement can be seen after gadolinium contrast injection (c) (Images courtesy of S. Dekeyzer, Aachen)

Fig. 21.15
Multicystic echinococcosis of the left thigh. Coronal T1-WI (a) and T2-WI (b) MRI shows multivesicular hydatidosis of adductor space
21.2.2.7 Other Inflammatory Myopathies
Inflammatory myopathies include focal myositis, nodular myositis, proliferative myositis, and diabetic muscle infarction. Clinically, inflammatory myopathies often present as a diffuse swelling of the thigh or calf, with or without tenderness. Only on rare occasions, they present as a solitary soft tissue mass. These different entities are only distinguishable by their histologic appearances [85], often requiring a biopsy for correct diagnosis.
On MR imaging studies, myopathies are characterized by non-focal hypointense areas on T1-weighted images and hyperintense signal on T2-weighted images [86]. These diffuse signal changes are even better seen when a T2-weighted fat suppression sequence is used [74]. Differential diagnosis includes infectious myositis, trauma, muscular denervation, muscular dystrophy (such as Duchenne’s [106] or Becker’s muscular dystrophy), rhabdomyolysis, polymyositis, dermatomyositis [74], and soft tissue malignancy. In most of these cases, clinical and laboratory tests will permit to make the correct diagnosis.
Focal Myositis. Focal myositis is a relative rare usually self-limiting soft tissue pseudotumor. It is usually found in the lower extremities, 50 % of the cases being located in the thigh and 25 % in the lower leg. Other more rare locations include the neck, tongue, perioral region, forearm, hand, abdomen, eyelids, and paraspinal muscles [94]. There is no sex or age predilection [94].
Typically, focal myositis presents as a local intramuscular soft tissue swelling, which can rapidly grow in a few weeks (Fig. 24.16). In more than 50 % of the cases, pain is the main symptom. Usually the process is limited to one muscle, but involvement of multiple muscles has been reported. One-third of the patients with focal myositis evolve to polymyositis or a polymyositis-like syndrome, suggesting that focal myositis is a localized form of polymyositis [14].
On MRI, focal myositis is of increased signal intensity on T2-weighted images, in one or more muscle groups. In contradistinction to most soft tissue sarcomas, the internal muscle bundle is spared (Fig. 21.16) [184].


Fig. 21.16
Focal myositis of the left rectus femoris muscle. (a) Axial FS T2-weighted MR image; (b) sagittal FS T2-weighted MR image. There is diffuse increased signal within the rectus femoris muscle with sparing of the individual muscle bundles
Diabetic Muscle Infarction. Diabetic muscle infarction is a rare complication of diabetes mellitus. Patients with poorly controlled type 1 insulin-dependent diabetes mellitus and severe end-organ damage are most frequently affected, although it may occur in a well-controlled patient without known diabetic complications [89]. Although the pathogenesis is still to be completely clarified, the most likely hypothesis is that the muscle infarction is secondary to vascular disease such as arteriosclerosis and diabetic microangiopathy [178], while some authors suggest an alteration in the coagulation-fibrinolysis system [140].
Diabetic muscle infarction typically presents as a sudden onset of severe pain in the thigh (especially in the quadriceps muscle) or calf, with diffuse enlargement of the involved muscle or muscle groups. After subsequent partial resolution, a painful palpable mass can be found in up to one-third of the cases [178]. Bilateral involvement has been described, with a reported frequency varying from 8 % to more than one-third of the cases [11, 89, 172, 178].
Clinically, it is frequently misdiagnosed as an abscess, neoplasm, or myositis, often requiring a biopsy for further evaluation [33, 47, 89]. Commonly there is elevated erythrocyte sedimentation rate, but no leucocytosis. This can be helpful in the differentiation from pyomyositis [89].
The diagnosis may be suspected on ultrasound if a well-marginated, hypoechoic intramuscular lesion with intralesional linear echogenic structures is present. There is typically absence of intralesional motion or swirling of fluid when pressure is applied with the transducer. This differentiates diabetic infarction from an abscess or a necrotic mass [47, 105].
MR images display enlargement of the involved muscles, with uniform increased signal intensity on T2-weighted and inversion recovery images demonstrating the edematous and inflammatory changes [11, 33, 86, 89]. T1-weighted images show normal or decreased signal intensity in the involved muscles, the swelling being sometimes less appreciated on this sequence [89].
Perifascial and subcutaneous edema are best evaluated on inversion recovery and fat-suppressed T2-weighted images [84]. Additionally, MRI can detect subclinical muscle infarction months before the onset of clinical symptoms [89].
Atypical presentations have been reported as a high signal of the affected muscle on T1-weighted images, presumably reflecting intramuscular hemorrhage [169].
21.2.2.8 Bursitis
Bursae are spaces near joints containing small amounts of fluid, reducing friction between different structures. The clinical most important are the trochanteric, subdeltoideal, ischiogluteal, pes anserina, iliopsoas, retrocalcaneal, and olecranon bursae, because they are the most commonly affected ones.
The amount of fluid may increase due to inflammation following overuse, direct trauma (rheumatoid), arthritis, or infection, resulting in a soft tissue mass. Imaging of bursitis is further discussed in Chap. 20.
21.2.2.9 Granulomatous Disorders
Tuberculous Infection of Soft Tissue
Tuberculous infection of soft tissue often accompanies involvement of lymph nodes, bones, or joints (Fig. 21.17) [174]. Without proper treatment, it can evolve to a cold abscess. A periosteal reaction in adjacent bone can sometimes be found [174].


Fig. 21.17
Tuberculous osteomyelitis involving the sternum and adjacent abscess: axial CT images (a, b) shows sternal osteolysis associated to pre- and retrosternal fluid collection with peripheral enhancement after intravenous contrast administration. Ultrasonography (c) shows sternal osteolysis with pre- and retrosternal fluid collection
Sarcoidosis
Sarcoidosis is a systemic granulomatous disorder which can affect multiple organs. Muscle involvement is rare and occurs in 1.4–6 % of patients with sarcoidosis [73, 136]. Three main clinical presentations of muscular sarcoidosis can be distinguished: an acute myositis, a diffuse atrophic form, and a nodular form [12, 134, 177]. Clinical symptoms are often absent.
The acute myositis type occurs exclusively in the early stage of sarcoidosis, presenting as myalgia secondary to inflammation. MR imaging is usually negative, presumably because of the sparse distribution and small size of epithelioid cell granulomas [135].
In the diffuse atrophic myopathic form, patients can present with myalgia, muscle weakness, and atrophy [134]. The muscles of de proximal portions of the extremities are frequently involved [117]. MR imaging findings are nonspecific revealing proximal muscle atrophy with fatty replacement [125]. Differentiation from a corticoid myopathy is mainly based on clinical and laboratory findings.
The least common form is the nodular presentation, presenting as a single or multiple sarcoid nodules (Fig. 21.18). They may or may not be clinically palpable. These nodules appear elongated and extend along muscle fibers [134]. On ultrasound examination, sarcoid nodules present with a hyperechoic center and a hypoechoic peripheral zone [134]. They may also present with well-defined borders and an overall hypoechogenic aspect [177].


Fig. 21.18
A 56-year-old woman with a tender palpable mass in the left calf: (a) axial spin echo T1-weighted MR image; (b) sagittal spin echo T1-weighted MR image with fat suppression; (c) axial spin echo T1-weighted MR image with fat suppression after intravenous contrast administration. A nodular lesion, slightly hyperintense compared to muscle, is shown on the axial T1-weighted image (a), with a central hypointense center presumably corresponding to fibrous tissue. After intravenous contrast administration, a homogeneous enhancement of these large and other smaller nodules is seen (c). The sagittal image further demonstrates the three layer composition, with a linear low-intensity stripe interposed between two high-intensity areas (b), longitudinally extending between normal muscle tissue. This case illustrates a rare soft tissue presentation of sarcoidosis
On MR imaging, the nodules may have a star-shaped hypointense center on all axial pulse sequences (“dark star” sign), which is believed to correspond with fibrous tissue and does not enhance after intravenous contrast administration [135, 136, 186, 199]. However, this central structure is not present in the acute stage of the disease. It can also be absent in small nodules (<10 mm), presumably because of the short time of granulomatous inflammation in these small structures.
The peripheral area of the nodules is slightly hyperintense compared to muscle on T1-weighted images, with homogeneous high signal intensity on T2-weighted images. There is homogeneous enhancement after intravenous contrast administration, secondary to the high cellularity of granulomas and edema [177].
Coronal and sagittal images may show the “three stripes” sign, consisting of a hypointense inner stripe and hyperintense outer stripes [134, 199]. However, after steroid therapy the sarcoid nodules may disappear [177], or only the inner stripe may be visualized [136].
Diffusion weighted imaging (DWI) may be useful to differentiate the two components of sarcoidosis, active inflammation and fibrosis. Areas of active inflammation are of high signal on DWI [166].
Very rarely periosteal reaction is seen in case of muscular sarcoidosis [176].
Cat Scratch Disease
Cat scratch disease is a benign, self-limiting cause of regional lymphadenitis affecting mostly children and young adults. In more than 90 % of the cases, there is a history of recent contact with cats, cat scratch, or both. In contrast, the site of inoculation is not always found. The Gram-negative bacillus Rochalimae henselae, also called Bartonella henselae, is the microorganism most often incriminated. In an otherwise healthy host, the adenitis resolves spontaneously within 3 weeks to several months, even without antibiotic therapy [49].
Cat scratch disease has a wide spectrum of clinical manifestations, ranging from regional lymphadenitis to disseminated infection. Regional lymph node enlargement occurs most commonly at the medial epitrochlear region of the elbow [119]. Other locations include the axilla, groin, and popliteal fossa [65, 164]. A typical case includes skin lesions and an associated enlarged painful reactive adenopathy commonly presenting along a single lymph node chain. Involved glands can have diameters up to 5 cm [112]. Multiple nodes at a single site may be involved as well. Disseminated infection is unusual (5–10 %) [112], most frequently seen in immune-compromised patients. Neurological involvement is uncommon [112].
The sonographic finding of an epitrochlear mass due to cat scratch disease most commonly consists of a hypoechoic lobular or oval mass with central hyperemia on power Doppler and a possible adjacent fluid collection. The adjacent or intranodal fluid collection is believed to correspond to nodal suppuration or necrosis. Asymmetrical shape and a hyperechoic hilum seem to differentiate cat scratch disease from other epitrochlear masses (including sarcoma, metastatic disease, or lymphoma) [119].
CT shows a soft tissue mass corresponding to involved lymph nodes. Central necrosis can be seen as a low attenuation [49, 191]. MR images reveal the regional lymphadenopathy as homogeneous or heterogenic masses surrounded by edema [49, 65]. T1-weighted images show a homogeneous isointense signal intensity compared to muscle (Fig. 21.19). On T2-weighted images the area of the mass and surrounding edema becomes hyperintense. After intravenous contrast injection, there may be homogeneous or slight peripheral enhancement of the involved lymph nodes and adjacent soft tissue edema [49, 65].


Fig. 21.19
Cat scratch disease in a 15-year-old boy: (a) axial spin echo T1-weighted MR image; (b) axial turbo spin echo T2-weighted MR image; (c) axial spin echo T1-weighted MR image after intravenous contrast administration. A round, well-defined lesion is located adjacent to the neurovascular bundle at the left elbow. On T1-weighted images (a) the lesion has slightly higher and relative homogeneous signal intensity compared to muscle, with a more intermediate signal intensity on T2-weighted images (b). After contrast administration there is moderate, mostly homogeneous enhancement of this mass
Bone involvement is rare, usually presenting as lytic lesions. A periosteal reaction and associated sclerosis can be found [80]. A single osteolytic lesion can simulate Langerhans cell histiocytosis [20].
The final diagnosis is based on polymerase chain reaction for DNA analysis or serologic testing. Culture of Bartonella species is difficult and usually unsuccessful. Histology reveals nonspecific granulomas with central necrosis at later stages [119].
Injection Granulomas
Injection granulomas are most often encountered in the upper outer quadrant of the buttocks and in the deltoid muscle [153]. They may be located either within the subcutaneous fat or gluteus muscles. The typical location and the absence of muscle distortion are the clues to the diagnosis. On plain films and CT subcutaneous injection, granulomas appear as small well-defined nodules, most often containing ringlike calcifications. These lesions are hypointense on T1-weighted MR images. On T2-weighted images, injection granulomas can be hyperintense or hypointense, depending on whether the major pattern of the lesion is inflammatory or fibrous. This pattern depends on the time elapsed after injection (Fig. 21.20). Intramuscular granulomas may have a linear pattern of calcification along the course of the muscle fibers.


Fig. 21.20
Typical subcutaneous injection granuloma in the left gluteal area: (a) plain radiograph of the left hip, (b) CT, (c) ultrasound, and (d) axial turbo spin echo T2-weighted MR image. A round, well-defined ringlike calcification lesion is seen within the subcutaneous tissue of the left gluteus region on plain films (a) and CT (b). On ultrasound, there is a linear reflection with the subcutis with retroacoustic shadow. (c) The lesion is of low signal intensity on T2-weighted images (d)
Mycetoma
Mycetoma is an implantation mycosis characterized by large tumorlike swellings and caused by actinomycetes (actinomycetoma) and fungi (eumycetoma) [161]. It is mostly located in the foot, responsible for 70 % of infections [60], but it has also been reported in hands, arms, legs, and back [3]. This disease is mainly found in tropical and subtropical regions of the world and the majority of patients are reported from Mexico, Senegal, Sudan, and India. The true prevalence and incidence are not well defined [160]. It usually affects young adults with male predominance. The causative agents are organisms living in the soil, and infection is generally acquired by an accidental painless inoculation through the skin by a thorn or splinter in barefoot individuals [55]. Incubation period varies from several weeks to months. Initially, the patient presents with a painless bump, which develops into a classic triad of chronic induration, draining sinuses, and fungal grain discharge. Early diagnosis is important because infection can invade deep tissues, muscle, bone, and even visceral organs, causing damage, deformity, or even death secondary to systemic spread [168, 192].
The radiologic findings include soft tissue swelling, periosteal reaction, cortical erosions, rounded lucencies (Fig. 21.21a), reactive sclerosis commonly affecting calcaneus and metatarsals, initially surrounding lucencies and then extend largely, joint destruction, osteopenia due to long-term immobilization, and bone lysis occurring in the late course of the disease [192]. If treatment is successful, the periosteal reaction coalesces, forming “melting snow” appearance on the cortex of small bones [151]. Ultrasonography (US) typically shows hypoechoic masses containing hyperechoic spots corresponding to fungal grains. This characteristic appearance on US is seen when fungal granules are of large size and previous to sinus formation. Computed tomography (Fig. 21.21b) is the preferred imaging tool to assess cortical erosions. The masses have similar density to muscles and fungal granules have a high density.


Fig. 21.21
Mycetoma (Madura foot) (a) lateral radiograph of the hindfoot showing soft tissue shadow (arrow) in the region of Kager fat without bone erosion. (b) Axial CT scan of the foot showing several rounded lesions (arrow) involving medial soft tissues of the foot with extrinsic bone erosions of the calcaneus (c) axial T1-weighted MR image of the ankle showing multiple inflammatory granulomata, appearing as conglomerates of small (2–5 mm) round hypointense lesions, several with “dot-in-circle sign” (arrows)
MRI (Figs. 21.21c and 21.22) can characterize the soft tissue masses of mycetoma, provide an early diagnosis, and assess the local extension of the infection. Mycetoma is characterized by the formation of microabscesses consisting of aggregates of the fungal granules (“sulfur granules”) and surrounded by granulation tissue. On MRI mycetoma appears as small, round hyperintense lesions (representing granulation tissue) measuring 2–5 mm, surrounded by a low signal intensity rim (representing fibrous septa). The central low signal intensity dot is the result of susceptibility artifact caused by the presence of a conglomeration of fungal grains. This finding was first reported by Sarris et al., as the “dot-in-circle” sign. This sign can also be demonstrated on T1-weighted images following intravenous gadolinium administration [163].


Fig. 21.22
Actinomycotic abscess in a 21-year-old man (a–c) and a 23-year-old man (d, e): (a) sagittal spin echo T1-weighted MR image; (b) sagittal T1-weighted MR image after gadolinium contrast injection; (c) axial T2-weighted MR image; (d) coronal spin echo T1-weighted MR image; (e) axial turbo spin echo T2-weighted MR image with fat suppression. In the first patient, there is a collar-button-like mass involving the right splenius and semispinalis muscles which is isointense to muscle on T1-weighted images (a) and hyperintense on T2-weighted images (c). After contrast injection there is moderate enhancement of the lesion without evidence of necrosis (b). After resection of the slowly growing mass, the lesion proved to be an actinomycotic abscess. In the second patient, the images show an ill-defined structure of homogeneous intermediate signal intensity on T1-weighted images (d) at the level of the paraspinal muscles on a low thoracic level. The lesion extends both cranially and caudally, invading the right psoas muscles and the spine (d, e)
Before sinus formation and discharge of fungal grains, early diagnostic may be difficult and mycetoma may be mistaken for a neoplasm (Kaposi sarcoma), neuropathic foot, or other chronic bacterial or tuberculosis infection [96, 208]. Biopsy and culture are required for the final diagnosis and identify causal agents to ensure correct antimicrobial therapy.
21.2.3 Traumatic Nerve Lesions Presenting as a Soft Tissue Mass
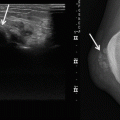
21.2.3.1 Morton’s Fibroma
The term Morton’s neuroma is a misnomer as it does not represent a true neuroma but rather perineural fibrosis and nerve degeneration, most likely due to repetitive compression and irritation of the interdigital nerve. Therefore, the term Morton’s fibroma is preferred [81].
Morton’s fibromas are most commonly found in the second and third intermetatarsal spaces and less frequently in the first and fourth. More than one intermetatarsal space may be affected as well. Morton’s fibroma is most commonly diagnosed in middle age, with a female predominance, and is believed to be related to the use of high-heeled shoes with increased weight bearing on the forefoot [196]. Although Morton’s fibroma is a common cause of intermetatarsalgia, in many cases it is not associated with clinical symptoms. Lesions with a transverse diameter smaller than 5 mm are often asymptomatic, as studies on healthy volunteers have shown, with a prevalence of small (<5 mm) Morton’s fibromas of approximately 30 % in asymptomatic individuals [19]. Lesions with a transverse diameter of 5 mm or more are most likely symptomatic.
Ultrasound examination is reliable and the most cost-effective imaging technique in detecting Morton’s fibroma [24]. A well-defined hypoechoic mass in the plantar soft tissues is seen at the level of the metatarsal heads (Fig. 22.23a). Dynamic ultrasound examination using Mulder’s test can increase conspicuity and diagnostic confidence. For this test, the patient’s forefoot is held in the sonographer’s non-imaging hand while performing lateral compression of the metatarsals and applying the transducer to the plantar aspect of the intermetatarsal regions. When this maneuver is performed on patients with a Morton’s fibroma, the mass is compressed between the metatarsal heads eliciting characteristic pain before it is plantarily displaced, often coinciding with a palpable click (Mulder’s sign) [9]. Elastography may be useful to detect smaller and less-defined lesions [133]. Although ultrasound has been proposed as a useful technique for guidance to inject corticosteroid, local anesthetic, or alcohol [9, 197], according to other authors, this technique seems not to improve the efficacy and safety of therapeutic injections if the clinician is well trained and is familiar with forefoot anatomy [109, 143].
Although in experienced hands, clinical examination and ultrasound may suffice for the diagnosis of Morton’s fibroma [24], MR imaging has been proven to be highly sensitive and specific in diagnosing Morton’s fibroma and may be used for difficult cases, differential diagnosis, and precise preoperative assessment [42, 203, 205]. On MRI, Morton’s fibroma appears typically as a tear-shaped, spindle-shaped, or dumbbell-shaped lesion in the region of the neurovascular bundle on the plantar side of the deep intermetatarsal ligament (Fig. 21.23). Morton’s fibromas display typical signal intensities on MR imaging sequences: isointense to muscle on T1-WI and hypointense relative to fat tissue on T2-WI [205]. There is no typical enhancement pattern, varying from low to moderate to marked enhancement. In the appropriate clinical setting, administration of gadolinium contrast medium is not required for a reliable diagnosis of a Morton’s fibroma. MR plays an important role in excluding other pathologies that can mimic Morton’s fibroma such as intermetatarsal or subcapitometatarsal bursitis (which occurs dorsal to the transverse metatarsal ligament), osteonecrosis of the metatarsal heads, metatarsophalangeal joint synovitis or dislocation, tendon sheath ganglion, pigmented villonodular synovitis, stress fractures, and other disorders associated with metatarsalgia [204, 205].


Fig. 21.23
Morton’s fibroma: (a) coronal T1-weighted MR image; (b) coronal FS T2-weighted MR image. (c) Corresponding coronal ultrasound at the forefoot. On T1-WI, a well-defined hypointense (isointense to muscle) lesion is seen at the intermetatarsal space between the third and fourth metatarsal head (black arrows) (a). The lesion is slightly heterogeneously hyperintense on FS T2-WI (white arrows) (b). On ultrasound the lesion is hypoechoic (c). The location and morphology of the tumor are characteristic of Morton’s neuroma
In most cases, therapy is conservative mainly comprising of footwear modifications, radiofrequency ablation, physical therapy, and local (corticosteroid and anesthetic) injections into the affected webspace. Surgical excision is only performed when necessary [9].
21.2.3.2 Traumatic Neuroma
Traumatic neuroma is a nonneoplastic reactive hyperplasia of nerve tissue and usually occurs at the proximal end of a nerve trunk that has been severed, partially transected, or injured as a result of trauma. Traumatic neuromas are classified into neuroma-in-continuity (NIC) after partial nerve transection or end-bulb neuromas (EBN) after complete disruption. EBNs lack distal continuity with the parent nerve, while NICs are contiguous both proximally and distally [2].
The most common location for traumatic neuromas is the lower extremity after amputation, followed by the head and neck, where they have been reported to occur after the extraction of teeth.
After limb amputation, neuromas may be asymptomatic when not compressed, but can cause unexplained pain and discomfort particularly when a prosthesis is worn. Physical examination may reveal a painful nodule at the site of the transected nerve, local tenderness over the injured nerve with distally radiating tingling (Tinel sign), denervation atrophy of the muscles, and sensory or trophic changes [38].
On imaging, traumatic neuroma may mimic a peripheral nerve sheath tumor (PNST). The history of a previous trauma is the key finding to the correct diagnosis.
Ultrasound reveals a hypoechoic mass at the distal end of site of the amputated nerve in case of an EBN [76]. In a transected nerve, ultrasound may reveal a focal gap due to nerve discontinuity and scar tissue at the level of transection and EBN formation at the proximal and distal stump margin [206]. An NIC is seen as a spindle-shaped mass on the course of the involved nerve and may mimic a PNST. Ultrasound-guided steroid or alcohol injection in painful stump neuroma has been reported as a successful method for pain relief [37, 104].
On MRI (Fig. 21.24), an EBN is seen as a T2 hyperintense nerve terminating in a baseball-shaped mass resembling a balloon on a string or a green onion appearance [2, 38]. To differentiate an NIC from a PNST, administration of gadolinium contrast is not useful as both lesions may demonstrate contrast enhancement. The lack of a target sign on T2-WI in a traumatic neuroma in conjunction with the clinical history of a previous trauma is the most useful clue to the correct diagnosis of a traumatic neuroma [2].
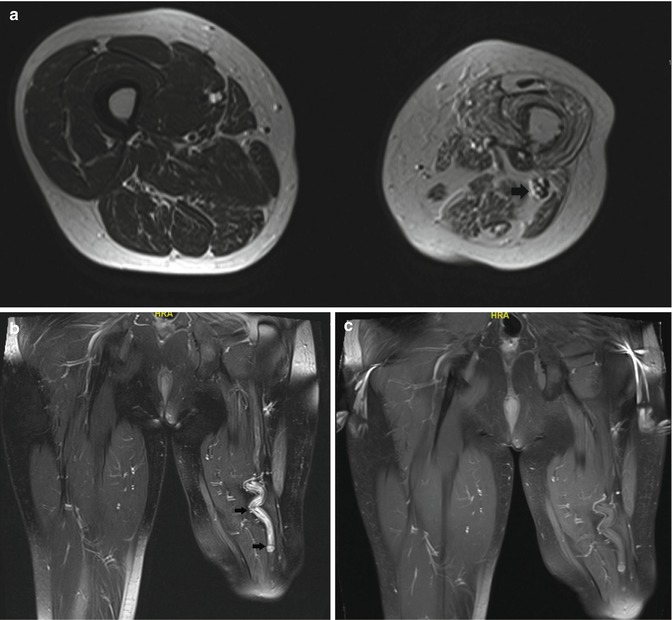

Fig. 21.24
Amputation neuroma of the left sciatic nerve in 46-year-old male: (a) axial T2-weighted MR image; (b) coronal FS T2-weighted MR image. (c) Coronal FS T1-WI following administration of gadolinium contrast. T2-WI. There is enlargement of the left sciatic nerve proximal to the site of amputation (arrows in a, b). Absence of significant enhancement of the sciatic nerve (c). Note muscle atrophy and fatty infiltration at the amputation stump
Distal denervation muscle atrophy serves as a useful secondary sign of nerve injury on MR imaging [38].
21.2.4 Other Posttraumatic Lesions
21.2.4.1 Hematoma and Contusion
A contusion is caused by a capillary rupture that provokes bleeding between the tissue muscle fibers, resulting in edema and inflammatory reaction. Contusions are not always painful, may present as a mass, and thereby cause clinical confusion. A soft tissue contusion appears on MR images as a diffuse interstitial infiltration due to edema. It is hyperintense on T2-weighted images but without architectural muscle distortion. However, the muscle may increase in volume, owing to inflammation. The history of a previous trauma is required to differentiate muscle contusion from focal myositis, which may have a similar imaging appearance.
The ultrasound appearance of hematomas is variable in time. Acute hematomas are hyperechoic and they become more hypoechoic with aging. They may have well-defined or irregular margins (Fig. 21.25a). Dynamic evaluation with muscle contraction is valuable for assessing disruption of muscle architecture. On CT, acute hematoma appears as a hyperdense area; MR imaging has replaced CT in the imaging of hematomas.
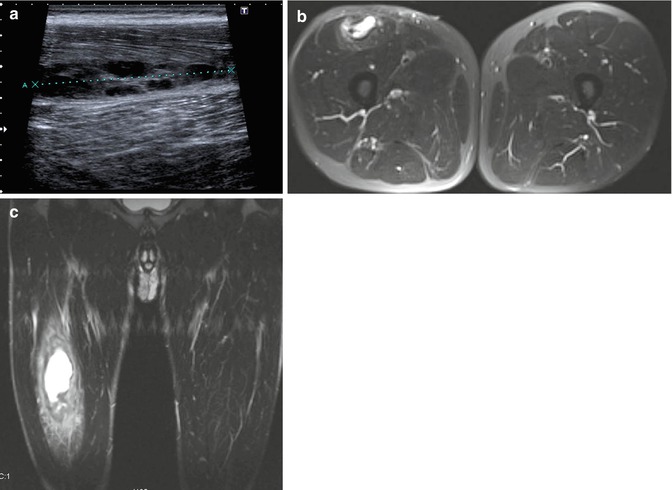

Fig. 21.25
Muscle hematoma in the right rectus femoris muscle. (a) Sagittal ultrasound shows focal disruption of the muscle fibers (hematoma); (b) axial FS T2-WI; (c) coronal FS T2-WI FS T2-WI. MRI confirms discontinuity of the right rectus femoris and the presence of a hyperintense hematoma with surrounding muscle edema
The MR imaging appearance of muscular hematomas (Figs. 21.25 and 21.26) reflects the pathophysiology of forming hemoglobin breakdown products, which are the main constituents of a hemorrhagic collection. Further discussion of the pathophysiology of hemoglobin degradation is beyond the scope of this chapter.
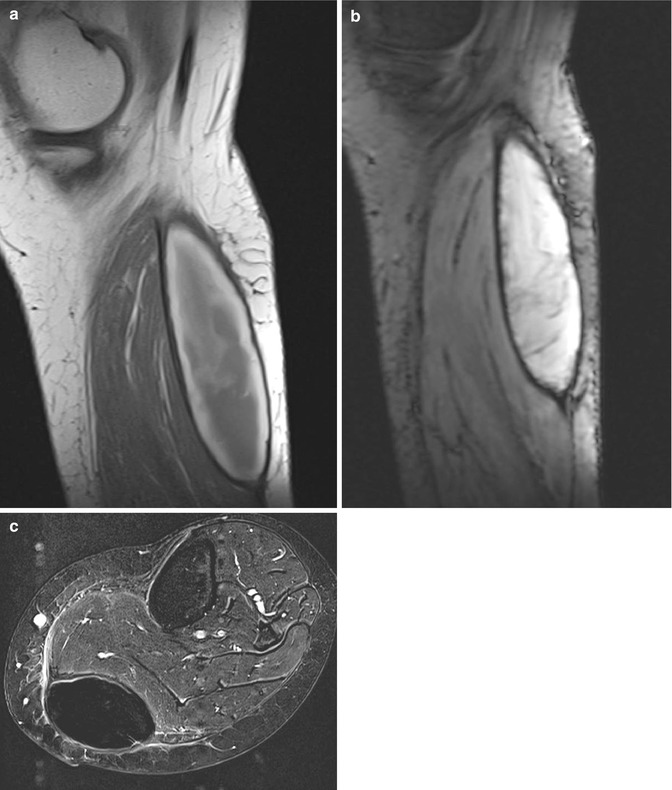

Fig. 21.26
Subacute hematoma of the calf: (a) sagittal spin echo T1-weighted MR image, (b) sagittal gradient MR image. There is a fusiform mass of intermediate signal intensity of the center, high signal intensity of the periphery, and adjacent subtle dark peripheral rim on T1-weighted images. The different layers are due to the presence of, respectively, intra- and extracellular methemoglobin and hemosiderin (a). On gradient images, overall signal intensity is very high, exception made for a low signal intensity peripheral rim caused by hemosiderin (b). Signal intensities are characteristic for a subacute hematoma. (c) Axial substraction image after injection of intravenous gadolinium contrast shows the absence of nodular enhancement which argues against the presence of underlying tumoral mass lesion
In an acute hematoma, the signal characteristics are dominated by intracellular deoxyhemoglobin. The lesion is iso- or slightly hypointense on T1-weighted images compared with muscle. Susceptibility effects also lead to low signal on T2-weighted images [28].
A hematoma in the early subacute stage is characterized by the presence of intracellular methemoglobin. This produces a high signal intensity on T1-weighted images, often visualized as a high-intensity peripheral rim. This high-intensity rim is a useful sign, as it may be the only clue that the mass is a hematoma. Susceptibility effects persist on T2-weighted images.
When loss of cell compartmentalization occurs in late subacute hematomas, extracellular methemoglobin results in Tl shortening. On T2-weighted images, the hematoma may be outlined by an area of high signal intensity (Fig. 21.25b-c). Diffuse edema is also present within the muscle in acute and subacute hematomas.
Finally, hemosiderin in a chronic hematoma also produces susceptibility effects on T2-weighted images. This results in low signal intensity on T1-weighted images and particularly on T2-weighted images. Blooming artifact is seen when using gradient echo imaging. Furthermore, this phenomenon is accelerated at the periphery of the collection, resulting in a peripheral hypointense rim, whereas the central portion of the hematoma may remain hyperintense [28].
It is important to differentiate hematoma from hemorrhagic tumor, because hemorrhage may obscure tumor tissue. T1-weighted images with fat suppression can aid in the differentiation, further discrimination methemoglobin from fatty tissue [39]. The best features suggesting hematoma are the progressive decrease in size of the lesion, the presence of fluid-fluid levels, and the time-dependent signal intensity changes. Conversely, the presence of enhancing nodules after contrast medium administration may suggest the presence of tumor [85]. Nevertheless, organized hematomas can show some enhancement.
When in doubt a biopsy should be performed to establish a confident diagnosis, especially if there is an increase in size of the hemorrhagic mass.
A chronic expanding hematoma is an entity characterized by its persistence and increasing size for more than 1 month after the initial hemorrhage [108]. MR reveals a heterogeneous signal intensity on both T1- and T2-weighted images, with a peripheral rim of low signal intensity [6]. Although the absence or presence of contrast enhancement is often used to distinguish a hematoma or a hemorrhagic neoplasm, intralesional nodular enhancement pattern may be seen in chronic expanding hematoma [108].
21.2.4.2 Foreign Body Reactions
Foreign bodies such as plastic, wood, glass, and silica may penetrate the soft tissues and produce an inflammatory reaction. Clinically, a foreign body reaction first appears as a painful soft tissue swelling, and after a quiescent period of weeks or months, the symptoms may reappear [175]. If not removed immediately, a foreign body can become encapsulated with fibrous tissue and form a granuloma. The presence of histiocytes and giant cells with a surrounding inflammatory reaction is useful in establishing the diagnosis.
Different imaging methods have each their advantages and limitations in the evaluation of foreign bodies. Radio-opaque bodies can be demonstrated on conventional radiographs. When the lesion is in or near the bone, radiographs can show osteolytic and/or osteoblastic bone lesions [99, 159]. Since a positive history of penetrating trauma is not always obvious, and some lesions can be radiolucent, a foreign body reaction can be mistaken for a neoplasm [99, 159].
Ultrasound is a primary imaging tool when conventional radiographs fail in detecting the foreign object. Its use in the detection and guided retrieval of a suspected foreign body has been well established [29]. Ultrasound shows large differences in acoustic impedance between hyperechoic foreign bodies and hypoechoic surrounding inflammatory tissue (Fig. 21.27).


Fig. 21.27
Inflammatory reaction surrounding a wooden splinter at the dorsal aspect of the hand. (a) Clinical picture of the right hand showing swelling of the dorsum of the hand. There are two more pronounced red noduli at the second metacarpal corresponding to focal abscesses. (b) Ultrasound showing the wooden splinter as a linear reflection with surrounding power Doppler signal in keeping with increased vascularity
On MR imaging studies, the foreign body itself is usually low, due to the presence of few mobile protons in the commonly found foreign materials (glass, wood, metal, etc.) [85, 99, 124, 182]. However, dispersed oil droplets can induce a granulomatous reaction and present as subcutaneous or intramuscular soft tissue masses with high signal on T1-weighted images [101]. An intralesional fat-fluid level may be seen at the interface of fatty and necrotic components in case of injection granulomas in bodybuilders. The combination of the clinical history and the typical location at usual locations for injection (such as the shoulder and gluteus muscles) are the clues to the correct diagnosis of these pseudotumoral soft tissue masses [8, 185].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree