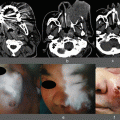
Fig. 18.1
Measurement of blood supply in the gastric tube by LDF. Whole gastric tube (a). Antrum of the gastric tube (b). Middle portion of the gastric tube (c). Top of the gastric tube (d). The right lower corner of each figure indicates the relative measurement data of LDF
18.1.3 Intraoperative Fluorescent Imaging
Recent advances have led to the development of intraoperative fluorescent imaging (IFI) using the SPYTM system, which allows the patency of a coronary artery bypass graft to be evaluated intraoperatively based on the detection of indocyanine green (ICG) fluorescence [6, 7]. We began using ICG fluorescence in July 2008 to visualize the blood supply to reconstructed organs during esophagectomy and found that it was very useful [8]. We have since accumulated more patients who underwent esophagectomy and esophageal reconstruction. In the present study, we reevaluated the efficacy of ICG fluorescence and reviewed a recent clinical evaluation of the ICG method in the field of esophagectomy.
18.2 Methods
18.2.1 Patient Characteristics
Sixty-two patients underwent esophagectomy for thoracic esophageal cancer, three were treated for cervical esophageal cancer, and five had double cancer in the thoracic and cervical regions (Table 18.1). There were 56 men and 14 women with an average age of 67 years (range, 44–86 years). Twenty-one patients received preoperative chemotherapy, two received preoperative chemoradiotherapy, and two had received radiotherapy several years before surgery. Three patients who had undergone jejunal graft reconstruction and two patients who had undergone esophageal bypass were evaluated at this time. Two patients who underwent partial resection for gastric tube cancer in the reconstructed gastric tube were also evaluated (Table 18.2).
Table 18.1
Characteristics of patients who underwent esophagectomy
Number | |
|---|---|
Age | 67 (44–86) |
Sex | |
Male | 56 |
Female | 14 |
Tumor locationa | |
PhMt | 3 |
CeMt | 2 |
Ce | 3 |
Ut | 3 |
Mt | 34 |
LtAe | 25 |
TNM stageb | |
1 | 18 |
2a | 11 |
2b | 8 |
3 | 30 |
4 | 3 |
Preoperative treatment | |
Chemotherapy | 21 |
Chemoradiotherapy | 2 |
Radiotherapy | 2 |
None | 45 |
Table 18.2
Characteristics of patients who underwent reconstructive surgery or resection for gastric tube cancer
Number | |
|---|---|
Age | 66 (45–79) |
Sex | |
Male | 6 |
Female | 1 |
Procedure | |
Bypass | 2 |
Free jejunal graft | 3 |
Partial resection of the gastric tube | 2 |
Preoperative treatment | |
Chemotherapy | 1 |
Chemoradiotherapy | 2 |
Radiotherapy | 0 |
None | 4 |
18.2.2 Operative Procedures
After esophagectomy, we made a gastric tube or colonic graft and pulled it up via the retrosternal, posterior mediastinal, or subcutaneous route depending on the patient. We routinely used the retrosternal route. A gastric tube was commonly fashioned with a width of 4 cm. Anastomosis was performed in the cervical region by hand sewing or using a circular stapler (25-mm EEA) [9]. We used a subcutaneous route for esophageal bypass. When a free jejunal graft was used, we first made a hand-sewn pharyngo-jejuno anastomosis, followed by microvascular anastomosis, and then jejuno-esophago anastomosis.
18.2.3 Modified Procedure
After preparing the gastric tube, the end of the short gastric vein was cut and we assessed the status of bleeding. Additional venous drainage was considered if bleeding was not continuous or very weak. We performed ICG fluorescence of the gastric tube in order to determine whether additional drainage was likely to be effective. If ICG fluorescence revealed a strong microvascular network, we concluded that the gastric tube did not need additional venous drainage or arterial anastomosis. If ICG fluorescence first appeared or became stronger after cutting the short gastric vein, we concluded that additional venous drainage would be effective. If ICG fluorescence did not appear after cutting the short gastric vein, additional arterial anastomosis was performed. If additional drainage or anastomosis was needed, anastomosis was performed between the short gastric vein or artery and the external cervical or superficial cervical vein [8].
18.2.4 ICG Imaging
Before and after pulling up the reconstructed organ, 2.5 mg of ICG dye (Diagnogreen; Dai-Ichi Pharm, Tokyo, Japan) was injected as a bolus. ICG fluorescence imaging was then performed using a near-infrared camera system (Photodynamic Eye; Hamamatsu Photonics K.K., Hamamatsu, Japan) and images were recorded. Briefly, images were obtained with a charge-coupled device (CCD) camera using a light-emitting diode with a wavelength of 760 nm as the light source and a filter to eliminate light with wavelengths below 820 nm before detection [10]. Images were sent to a digital video processor and displayed on a monitor. [8]. In the case of gastric tube cancer, ICG fluorescence was performed before and after partial resection of the gastric tube.
18.3 Results
18.3.1 Operative Procedures
Twenty-six patients underwent thoracoscopic-assisted right thoracotomy in the left lateral position, one underwent left thoracotomy because of a right aortic arch, 41 underwent esophagectomy in the prone position, and two underwent cervical esophagectomy in the supine position. Regarding the method used to reconstruct the esophagus, a gastric tube was employed in 57 patients, gastric tube plus free jejunal graft in two patients, free jejunal graft in two patients, and colonic graft in nine patients. Reconstruction was performed via the posterior mediastinal route in two patients, by the subcutaneous route in 15 patients, and the retrosternal route in 51 patients (Table 18.3).
Table 18.3
Operative procedures performed on patients
Number | |
|---|---|
Method of esophagectomy | |
VATS (right thoracotomy) | 26 |
VATS (left thoracotomy) | 1 |
Prone position esophagectomy | 41 |
Cervical esophagectomy | 2 |
Reconstruction organ | |
Gastric tube
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|