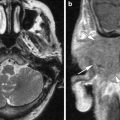
Fig. 1
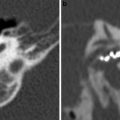
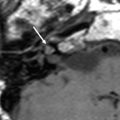
a Axial contrast-enhanced T1-weighted MR image shows an enhancing squamous cell carcinoma along the right EAC (arrow). b Axial and c coronal CT images show underlying osseous destruction of the right EAC (arrow). d Axial contrast-enhanced CT image shows an enlarged right parotid node (arrow), the first echelon node of EAC carcinoma
Diagnosis EAC carcinoma may be delayed as the initial presenting symptoms of otorrhoea and otalgia often mimic otitis externa. In addition, even the histological samples may sometimes be confused with pseudoepitheliomatous hyperplasia (Gacek et al. 1998). Essentially, any EAC lesion associated with bony destruction should be considered malignant until proven otherwise.
5 Middle Ear-Mastoid Complex
The middle ear is connected to the mastoid via the aditus ad antrum. Tumours of the middle ear may grow freely through the air spaces of the middle ear and mastoid. They can spread to other parts of the temporal bone via the oval and round windows as well as through the tympanic membrane. Other potential pathways beyond the middle ear-mastoid complex include the eustachian tube and along neurovascular structures into the nasopharynx, infratemporal fossa or neck. Aggressive tumours may erode directly through bone, particularly through the thin tegmen tympani or sigmoid plate into the middle or posterior cranial fossa and sigmoid sinus.
5.1 Glomus Tympanicum Paraganglioma
The most common benign tumour of the middle ear and mastoid is the glomus tympanicum paraganglioma (O’Leary et al. 1991). Paragangliomas are neuroendocrine tumours, originating from chromaffin cells in paraganglia or chromaffin-negative glomus cells derived from the embryonic neural crest. In the middle ear, these cells are found anywhere along the course of Jacobson’s nerve (the tympanic branch of the glossopharyngeal nerve) which enters the middle ear through the tympanic canaliculus and forms the tympanic plexus, which ramifies on the surface of the cochlear promontory and medial wall of the middle ear. The term glomus jugulotympanicum refers to paragangliomas that involve both the jugular foramen and middle ear. These tumours usually arise from glomus cells derived either from Jacobson’s nerve or Arnold’s nerve (the mastoid or auricular branch of the vagus nerve). Peak incidence is in the fifth and sixth decades and there is a clear female predominance (3:1).
The most common presenting symptom is pulsatile tinnitus. If the tumour is large, it may cause conductive hearing loss. Facial nerve palsy, sensorineural hearing loss or vertigo may result if the facial nerve or inner ear becomes involved. Other cranial nerve palsies may result from extension to the jugular foramen or hypoglossal canal. Occasionally, they may be asymptomatic. On clinical examination, a vascular retrotympanic mass is seen. The symptoms and clinical findings of a glomus tympanicum tumour may be indistinguishable from glomus jugulotympanicum, aberrant internal carotid artery or a dehiscent, high-riding jugular bulb and imaging is required to differentiate these entities. On high resolution CT, a nodular soft tissue mass is typically seen in the hypotympanum near the cochlear promontory (Fig. 2). The mass may fill the middle ear cavity but generally spares the ossicles. Bone erosion is usually not a feature, even in large tumours. Tumours may fill the epitympanum, attic and antrum, resulting in fluid retention in the mastoid. On MRI, the mass is seen as a strongly enhancing lesion within the signal void of the middle ear-mastoid complex, on T1-weighted post-contrast sequences, with variable T2-weighted signal. Larger tumours may exhibit the characteristic ‘salt and pepper’ appearance on T1-weighted sequences, with the ‘pepper’ representing hypointense signal flow voids caused by the large feeding arteries and the ‘salt’ representing subacute haemorrhage within the tumour.
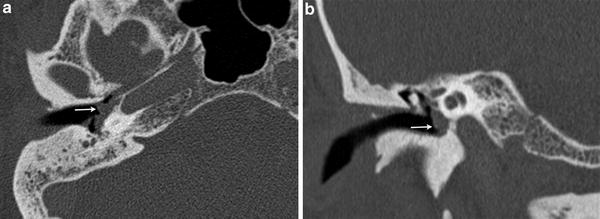

Fig. 2
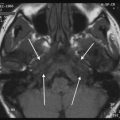
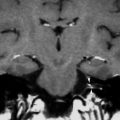
A 49 year-old female with pulsatile tinnitus and a red retrotympanic mass. a Axial temporal bone CT image shows a soft tissue tympanic mass over the cochlear promontory (arrow). b Coronal temporal bone CT image shows the tumour in the meso- and hypotympanum bulging against the lower tympanic membrane (arrow). The clinical and imaging features are consistent with a glomus tympanicum paraganglioma
5.2 Middle Ear Schwannoma
The next most common tumour involving the middle ear is the schwannoma. The most common schwannoma of the middle ear arises from the facial nerve, though it may arise from any nerve of the middle ear, including Jacobson’s nerve and Arnold’s nerve (Aydin et al. 2000; Wiet et al. 1985). On clinical examination, schwannomas may appear as a fleshy-white mass behind an intact tympanic membrane. On imaging, a well-circumscribed lobulated mass may be seen arising from the tympanic or mastoid segments of the facial nerve (Fig. 3) or lying separate from the facial nerve canal when the facial nerve is not involved. Post-contrast T1-weighted MRI shows enhancement of the mass.
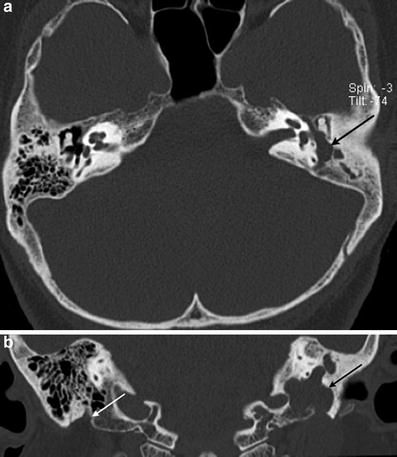

Fig. 3
Facial nerve schwannoma. a Axial temporal bone CT image shows a facial nerve schwannoma enlarging the posterior tympanic segment of the left facial nerve canal (arrow). b Coronal temporal bone CT image shows the schwannoma extending along the mastoid segment of the left facial nerve canal (black arrow). Note the normal right mastoid facial nerve canal, which opens through the stylomastoid foramen (white arrow)
5.3 Other Middle Ear Tumours
Congenital cholesteatomas are the next most common differential for a middle ear mass. Congenital cholesteatomas typically present with a whitish mass seen behind an intact tympanic membrane, as opposed to acquired cholesteatomas, which arise from a perforation or retraction pocket in the tympanic membrane and are associated with a history of chronic otorrhoea and recurrent middle ear infection. Congenital cholesteatomas arise when there is aberrant migration of external canal ectoderm beyond the tympanic ring. As a result, the tympanic membrane is intact. Both congenital and acquired cholesteatomas form as a result of the accumulation of exfoliated keratinous material in a sac lined by squamous epithelial cells. On CT, congenital cholesteatomas appear as a nodular soft tissue mass in the hypo- or mesotympanum, with or without ossicular erosion. Prussak’s space and the scutum are typically normal. On MRI, congenital cholesteatomas demonstrate intermediate signal intensity on T2-weighted and hypointense signal intensity on T1-weighted sequences without any enhancement post contrast. Rim enhancement is occasionally seen due to surrounding granulation tissue. Diffusion weighted MRI (DWI) may be used to differentiate congenital cholesteatoma from other middle ear masses, as cholesteatomas appear hyperintense on B1000 DWI sequences. Non-echoplanar sequences are preferred, as they allow for thinner sections and are less sensitive to susceptibility artefacts at the brain-bone interface (De Foer et al. 2006; Schwartz et al. 2011).
The middle ear adenoma is a rare tumour of the middle ear, considered by some to encompass a spectrum of histological types ranging from adenoma to carcinoid, depending on the degree of glandular or neuroendocrine differentiation (Torske et al. 2002). Middle ear adenomas most commonly present with conductive hearing loss and a soft tissue mass behind an intact tympanic membrane. On CT, they appear as a soft tissue mass engulfing the ossicles, indistinguishable from the far more common congenital cholesteatoma. Larger tumours may cause ossicular erosion. On MRI, they appear as an enhancing mass not confined to the cochlear promontory and may mimic glomus tympanicum (Zan et al. 2009).
Malignant tumours of the middle ear are rare. The most common malignant middle ear tumour is SCC, which is thought to arise secondary to squamous metaplasia, mainly in the setting of chronic otitis media (Fig. 4). A high prevalence of human papilloma virus (HPV) has also been reported in middle ear SCC associated with chronic otitis media (Jin et al. 1997).
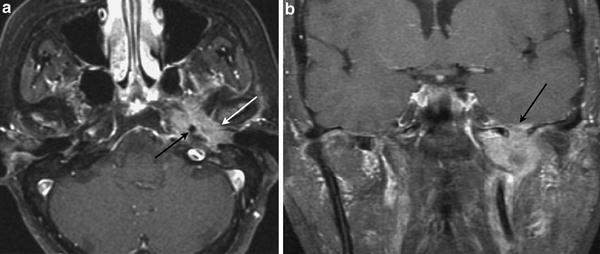

Fig. 4
Carcinoma of the middle ear. a Axial contrast-enhanced T1-weighted MR image shows a heterogeneously enhancing tumour arising from the left middle ear (white arrow), invading the petrous apex and surrounding the left petrous internal carotid artery (black arrow). b Coronal contrast-enhanced T1-weighted MR image shows superior spread with intracranial tumour extension (arrow)
Rhabdomyosarcomas are the most common paediatric soft tissue sarcoma and most common middle ear malignancy in children, although the temporal bone is an uncommon site of involvement (Adrassy 1997). Initially, the tumour may present as chronic otitis media, unresponsive to antibiotics. Cranial nerve dysfunction is common, particularly facial nerve palsy. CT may demonstrate a soft tissue mass with extensive bony destruction. MRI is useful to delineate the extent of the tumour, the intracranial extent and to differentiate tumour from fluid, although findings are non-specific. Rhabdomyosarcomas are typically isointense to muscle on T1-weighted, iso- to slightly hyperintense to muscle on T2-weighted and diffusely enhancing on contrast enhanced T1-weighted sequences. Langerhans cell histiocytosis (LCH) is the main differential for a soft tissue mass with aggressive bony destruction in a child. LCH may indistinguishable from rhabdomyosarcoma on imaging and biopsy is often necessary. Acute coalescent otomastoiditis may also be associated with soft tissue density in the middle ear and bony destruction, however patients are usually acutely ill and respond to antibiotics.
6 Inner Ear and Internal Auditory Canal
The inner ear comprises the membranous labyrinth bathed in perilymph within the bony labyrinth. The membranous labyrinth is a closed system of endolymph-filled tubes and chambers, which includes the vestibule (utricle and saccule), the semicircular ducts, the cochlear duct (scala media of cochlea), the endolymphatic duct and sac. The endolymphatic duct arises from the utriculosaccular duct (which connects the utricle and the saccule), and passes within the bony vestibular aqueduct to the endolymphatic sac which is lodged at the fovea of the vestibular aqueduct along the posterior wall of the temporal bone.
The IAC is approximately 10 mm long and transmits the facial and vestibulocochlear nerves between the posterior cranial fossa and the inner ear. The canal is divided by a horizontal crest of bone, the crista falciformis into an upper compartment (which houses the facial nerve anteriorly and the superior vestibular nerve posteriorly) and a lower compartment (which contains the cochlear nerve anteriorly and the inferior vestibular nerve posteriorly). The facial and superior vestibular nerves are further divided by a vertical Bill bar, although this small bony structure is not visible on imaging.
6.1 Schwannoma
Schwannomas are benign encapsulated tumours arising from the Schwann cells that wrap around cranial nerves. Histologically, they comprise of differentiated Schwann cells forming Antoni A (areas of compact spindle cells) and B patterns (areas of loosely arranged matrix with lipid-laden cells and cysts). Malignant schwannomas are very rare but have been reported (Balasubramaniam 1999).
6.1.1 Intracanalicular Schwannoma
It is postulated that schwannomas in the IAC most commonly arise from the vestibular division of the vestibulocochlear nerve (CN8) at the glial-Schwann cell junction, which is usually near the porous acousticus and hence their common presentation as a combined cerebellopontine angle (CPA)-IAC mass. However, there are also lesions that are entirely intracanalicular and it has been hypothesised that some of these tumours may have originated from the Scarpa’s ganglion on the vestibular nerve near the fundus of the IAC (De Foer et al. 2010).
The peak age for vestibular schwannomas is between 40 and 60 years old. Patients most often complain of unilateral progressive sensorineural hearing loss, sometimes with tinnitus and vertigo. On MR imaging, this tumour appears as an avidly enhancing cylindrical (intracanalicular schwannoma) or “ice cream on cone” (CPA-IAC schwannoma) mass (Fig. 5). Although intramural cysts are not uncommon in larger schwannomas (up to 25 %), they are hardly seen in the smaller intracanalicular lesions. Haemorrhagic schwannomas are rare (0.5 %). On imaging, it is important to look for any negative prognostic features for hearing preservation following surgery, which include a tumour size larger than 2 cm and tumour involvement of the IAC fundus or cochlear aperture (Somers et al. 2001).
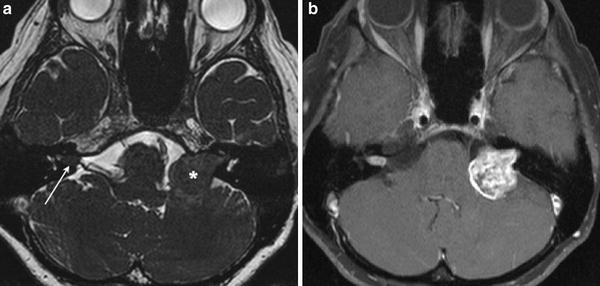

Fig. 5
a Axial high-resolution heavily T2-weighted MR image shows a cylindrical right intracanalicular vestibular schwannoma, and an “ice cream on cone” left CPA-IAC vestibular schwannoma. b Axial contrast-enhanced fat-saturated T1-weighted MR image shows avid enhancement in both schwannomas, with intramural cystic changes in the larger left-sided lesion. Bilateral vestibular schwannomas are hallmark of type 2 neurofibromatosis
Although a well-circumscribed IAC lesion should be considered a vestibular schwannoma unless proven otherwise, a few differential diagnoses exist.
An IAC facial nerve schwannoma may mimic a vestibular schwannoma clinically, although facial nerve (CN7) palsy is more common in the former (up to 73 %) (Ulku et al. 2004). If the tumour is confined within the IAC, it is indistinguishable from a vestibular schwannoma on imaging. An imaging diagnosis of facial nerve schwannoma is achieved when the tumour is seen extending into the labyrinthine segment of the facial nerve, giving rise to a “labyrinthine tail” detected on contrast-enhanced MRI.
While CPA-IAC meningiomas account for 5–8 % of all intracranial meningiomas, meningiomas arising from and confined to the IAC are rare with only a handful of case reports in the literature (Bohrer and Chole 1996; Langman et al. 1990). The clinical presentations of intracanalicular meningiomas are similar to those of vestibular schwannomas (up to 80 % with unilateral progressive sensorineural hearing loss and 50 % with tinnitus), although they tend to become symptomatic earlier (Laudadio et al. 2004). The imaging features often associated with other intracranial meningiomas, such as intramural calcification, dural tail and bone hyperostosis have never been described in all the intracanalicular meningiomas documented in the literature. As a result, they appear identical to IAC vestibular schwannomas on imaging and the definitive diagnosis requires histological confirmation.
Intracanalicular lipomas are rare congenital lesions believed to arise from maldifferentiation of meningeal precursor tissue (meninx primitiva). They are seen as non-enhancing IAC lesions with homogeneous hyperintense signal on T1-weighted MR imaging, which is clearly suppressed on fat-saturated sequence (Bonneville et al. 2007) (Fig. 6). A concurrent intravestibular lipoma may be present. Most of the IAC lipomas are discovered incidentally on MR imaging of the temporal bones (Swartz 2008). They usually incorporate CN7 and CN8 with dense adhesion, and should be considered “leave me alone” lesions as any surgical interventions might do more harm than good (De Foer et al. 2010).
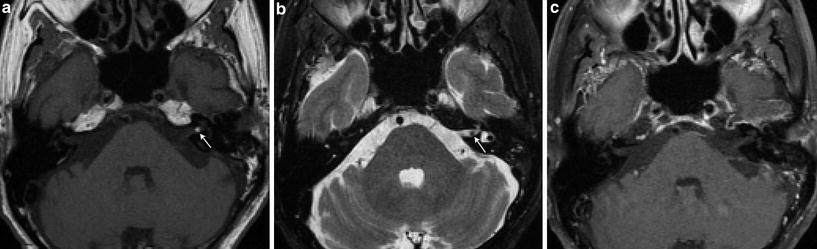

Fig. 6
Internal auditory canal lipoma. a Axial T1-weighted MR image shows a small hyperintense left intracanalicular lesion (arrow). b Axial T2-weighted fat-saturated MR image shows signal suppression of the lesion, which appears as a “filling defect” in the left IAC surrounded by the high signal cerebrospinal fluid (arrow). c Axial contrast-enhanced fat-saturated T1-weighted MR image shows no enhancement of the lesion, in keeping with a lipoma
6.1.2 Intralabyrinthine Schwannoma
Intralabyrinthine schwannomas originate from the perineural Schwann cells of the intralabyrinthine branches of the CN8. Approximately 80 % of these tumours are confined to the cochlea, often located anteriorly between the basal and second turns (Tieleman et al. 2008). Intralabyrinthine schwannomas may grow from the cochlea into the vestibule, and vice versa. They may also extend from the cochlea (transmodiolar) or vestibule (transmacular) into the fundus of the IAC.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree