Fig. 1
a Schematic drawing of a cochlear implant showing the externally worn speech processor and microphone, which is magnetically attached to a subcutaneous implanted receiver. The receiver contains electronics to decode the signals and generate electrical stimuli. An electrode array is connected to this receiver and inserted into the scala tympani of the cochlea. The external (b) and internal (c) components of a cochlear implant (Advanced Bionics Hires 90K and Harmony speech processor) are shown. The behind the ear part (b) contains the speech processor and a built-in microphone. Alternatively, a body worn processor can be used. The internal components consist of the magnet, receiver, and electrode lead. Implants of other manufacturers have a slightly different appearance, but contain the same components
Whether a hearing impaired person is a suitable candidate who would benefit from cochlear implantation should be examined by a multidisciplinary team, based on medical history, clinical, audiological, and imaging findings as well as the social and psychological setting. Over the last years, criteria for cochlear implantation candidacy have expanded considerably. There has been a shift toward implantation at a younger age. Prelingual adults who have never had a hearing experience may receive implants. Malformed inner ears, be it nonsyndromic or syndromic, or ossified cochleae as a result of labyrinthitis are no longer considered contraindications for implantation. Since the success of implantations is, among other factors, determined by the physiology and anatomy of the inner ear and auditory pathway, imaging plays an important role in the assessment of cochlear implant candidates.
2 Preoperative Assessment in Cochlear Implant Candidates
There are many causes of sensorineural hearing loss. They include genetic conditions, infectious or inflammatory diseases, traumatic sequelae, ischemic events, and toxic agents. Imaging may establish the cause of deafness, but in many cases no structural abnormalities can be found.
Systematic analysis for developmental or obliterative inner ear disease, abnormalities of bone metabolism, retrocochlear pathology, and anatomic variants of the middle ear will render information on feasibility of the procedure, preferred surgical approach including operation side, and type of cochlear implant that should be chosen.
2.1 Imaging Modalities
Both computed tomography (CT) and magnetic resonance imaging (MRI) render complementary information in the preoperative assessment of cochlear implant candidates. CT is an excellent modality to identify anomalies of the bony labyrinth, vestibular aqueduct, otic capsule, and eventual concomitant middle ear anomalies or anatomic variations that may complicate surgery. MRI is superior in the assessment of the membranous labyrinth, internal auditory canal (IAC), cerebellopontine angle (CPA) cistern, and auditory pathway. Contrast administration will increase the detection of infectious, inflammatory, or autoimmune diseases of the inner ear and of meningeal pathology as a cause of hearing loss. These findings may directly impact patient management (Hegarty et al. 2002). If only one imaging modality is chosen, one should bear in mind that abnormalities detected with MRI are more likely to influence the implantation process (e.g., asymmetric nerve hypo- or aplasia, cochlear obstruction) (Parry et al. 2005).
2.2 Inner Ear Malformations
Congenital hearing loss or profound hearing loss in early childhood may be the result of developmental abnormalities of the cochleovestibular system. They may be part of a syndrome or due to sporadic chromosomal abnormalities, which may be linked to gestational infections or ototoxic drugs. As described in Congenital Malformations of the Temporal Bone, arrested or aberrant development of the labyrinth will lead to a myriad of malformations.
Labyrinthine or cochlear aplasias are absolute contraindications for CI. In other dysplasia CI may be considered, but decision-making should be taking into account the neural function of the auditory nerve and—particularly in syndromic cases—cognitive function. Prior documentation of hearing or proven auditory nerve function is considered beneficial. Surgery may be technically challenging, especially in syndromic cases with associated middle ear anomalies where normal anatomical landmarks such as the facial recess, stapes, or round window are distorted or absent. A precise description of the middle and inner ear status on imaging will render a roadmap for the surgeon. Moreover, imaging will identify anomalies which predispose to perilymph gusher or perilymph oozing. “Gushing” of cerebrospinal fluid (CSF) during cochleostomy is the result of perilymphatic hydrops due to a broad communication between the subarachnoid space and the perilymph in the cochlea as can be seen in X-linked hearing loss (Friedman et al. 1997). Preoperative perilymph oozing or slow, persistent flow of CSF may occur in patients with enlarged vestibular aqueducts and modiolar deficiency. In case of asymmetric developmental abnormalities, imaging might influence the choice of side of operation (Fig. 2).
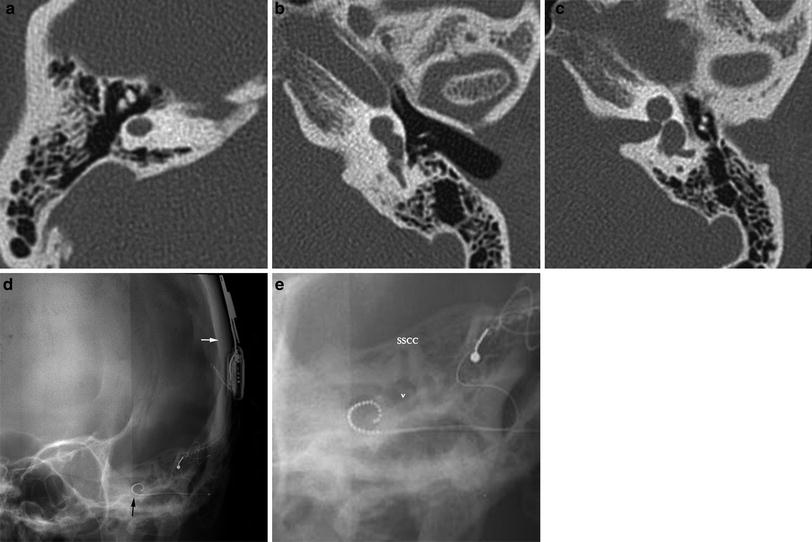

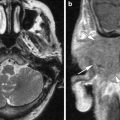
Fig. 2
Asymmetric congenital malformation determining side of operation: in this patient with congenital deafness on the right and profound sensorineural hearing loss on the left axial CT shows bilateral developmental abnormalities. On the right (a) labyrinthine aplasia precludes cochlear implantation. On the left (b, c) a small, cystic cochlea is present, which was successfully implanted. On a postoperative Stenvers view (d), the subcutaneously implanted receiver (white arrow) and the attached electrode lead with the cochlear implant (black arrow) is seen. On a magnified image e it is clearly seen that the electrode array projects medial to the promontory, which can be identified at the air-bone interface beneath the vestibulum (v) (SSCC superior semicircular canal)
2.3 Labyrinthitis
Labyrinthitis due to bacterial meningitis is the most common cause of postnatally acquired hearing loss. Other causes of labyrinthitis are viral infections, inflammatory conditions, trauma, and autoimmune diseases. (see also Inner Ear Pathology). In both infectious and noninfectious labyrinthitis, the labyrinth will become inflamed—leading to degeneration of sensory structures in the organ of Corti and of spiral ganglion cells. (Merchant and Gopen 1996; Rappaport et al. 1999; Klein et al. 2003). Eventually, labyrinthitis ossificans may develop with replacement of intralabyrinthine fluid by fibrous tissue or bone formation. This will increase the risk for complications during cochlear implantation or may even preclude implantation. Fibrous tissue may be removed preoperatively relatively easily with a little hook. Proximal bony obliteration may be drilled out increasing the surgical risk of damaging neural structures. Once the obliteration exceeds to the second half of the basal turn of the cochlea, surgical access to the scala tympani is precluded. In such cases, implantation of a double array may be considered. (Lenarz et al. 2001; Bredberg et al. 1997). If the ossificiation is limited to the scala tympani, a full insertion of the implant may be achieved into the scala vestibuli (Lin 2006) (Fig. 3e, f).
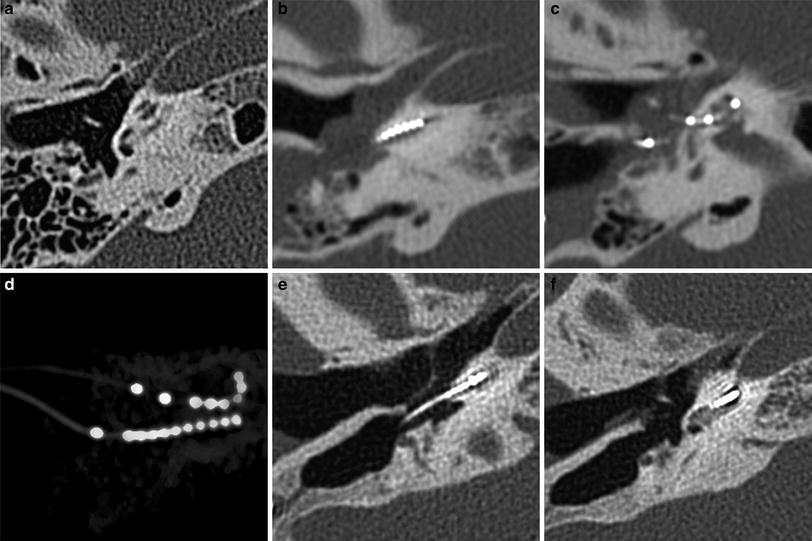

Fig. 3
Modified surgical approaches in labyrinthitis ossificans: Patient 1 (a–d) had a history of meningogenic labyrinthitis. Preoperative CT (a) shows extensive ossification in the basal turn of the cochlea, but a patent second and apical turn. a a double array was placed: the first array (b) is implanted into the proximal basal turn after drill-out. The second array (c) is directly inserted into the second turn of the cochlea through a second cochleostomy. Volume rendering image (d) illustrates the position of both arrays. In patient 2 (e, f) a scala vestibuli insertion was performed because of severe ossification of the scala tympani as seen in f
Labyrinthitis may progress rapidly to a fibro-osseous stage. Therefore, early audiometric testing and imaging are recommended and early intervention may be appropriate to improve the outcome in terms of hearing restoration. Bilateral implantation has been advocated by some groups. (Merkus et al. 2010).
Imaging findings will play a major role in treatment planning. Since MRI is sensitive to soft tissue changes in the early phase of labyrinthitis, it is the modality of choice in suspected acute labyrinthitis. In the acute phase of labyrinthitis, MRI will first show enhancement within the labyrinth without any signs of obliteration on T2 weighted images. As soon as fibroblastic proliferation occurs, T2 weighted images will show signal loss within the labyrinth. This fibrous tissue may or may not be enhancing. CT will frequently be normal in acute labyrinthitis or in case of purely fibrous replacement. Once neo-ossification occurs, CT will show osseous obliteration. The combination of MRI- and CT-findings will allow for differentiation between fibrous tissue and ossification (Fig 4).
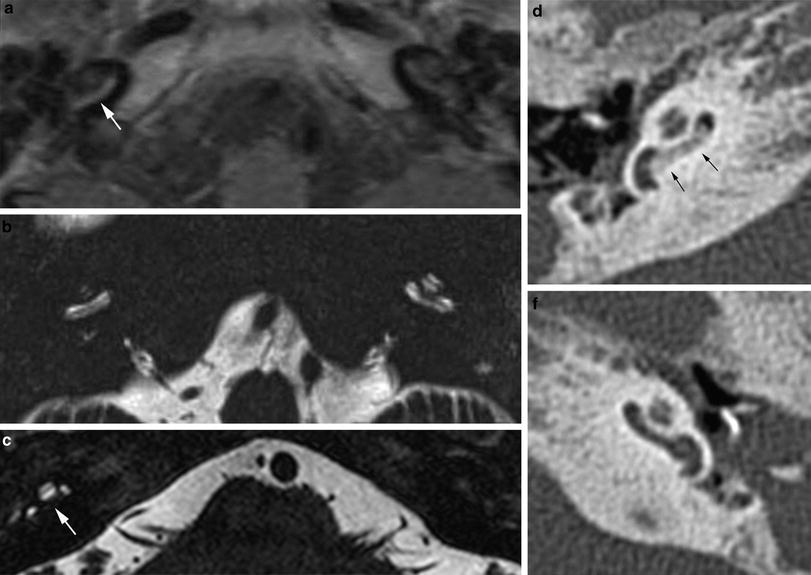

Fig. 4
MRI features of labyrinthitis ossificans: Patient 1 (a, b) developed hearing loss during meningitis. On contrast-enhanced T1 weighted MRI (a), enhancement of the basal turn of the cochlea is seen (arrow). A heavily T2 weighted image (b) shows normal signal intensity within the cochlea. This pattern indicates labyrinthitis in its acute stage where inflammatory cells fill the perilymfatic spaces. This may rapidly progress to the obliterative stage, where fibroblastic proliferation and ossification will occur as seen in the second patient (c–e). T2 weighted MRI shows signal loss in the scala tympani on the right (white arrow) and complete signal loss of the membranous labyrinth on the left. On a corresponding axial CT-image, this is shown to be due to bone formation within the cochlea on the right (black arrows in d) and fibrosis on the left (e)
The precise description of presence, location, and extent of obliteration of the cochlea will guide surgical planning as well as the choice of cochlear implant (single array, double array).
2.4 Otosclerosis and Other Bony Dysplasia
2.4.1 Otosclerosis
Otosclerosis usually causes mixed hearing loss and primary surgical treatment consists of stapes surgery. In advanced cases, however, cochlear implantation may be considered.
Otosclerosis is a disease of the otic capsule causing a cycle of bony changes leading to osteolytic foci and bone productive changes. The round window may become narrowed and sometimes abnormal bone formation to the cochlear lumen can be seen (Fig. 5). Ossification of the cochlea may also be seen as a complication after stapes surgery. Due to the altered, brittle bone, and anatomic distortions, the risk for complications during implant surgery increases (Rotteveel et al. 2004). Malpositioning of the cochlear implant may occur. Extensive osteolytic lesions or pathologic fractures due to manipulation may lead to CSF-leak. Postoperatively, the patients may experience stimulation of the facial nerve by sound. This is commonly thought to occur due to current spread toward the facial nerve through abnormal bone, but a recent study argued that rather than a decreased facial nerve threshold, increased levels for cochlear stimulation due to more current flowing out of the cochlea are responsible for this phenomenon. Fortunately, it is less frequently seen nowadays, as particularly perimodiolar designs with more shielding against lateral spread of current reduce the likelihood of facial nerve stimulation (Frijns et al. 2009).
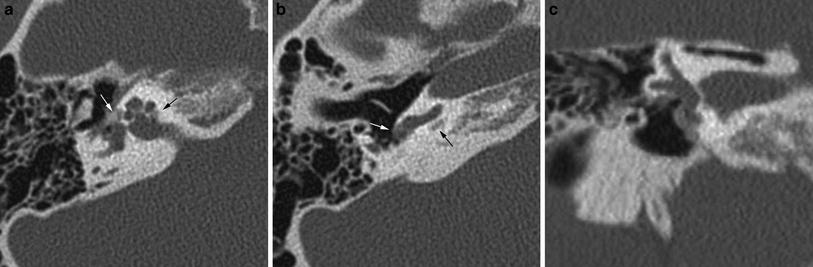

Fig. 5
Fenestral and retrofenestral otosclerosis with round window stenosis: on axial CT (a) an otospongiotic focus is seen at the fissula ante fenestram (white arrow) as well as posterior to the cochlea, extending into the internal auditory canal (black arrow). An axial image slightly more caudal to a shows narrowing of the round window niche due to an otospongiotic focus (white arrow), which obscures the surgical access route. Note also the lytic area posterior to the basal turn of the cochlea (black arrow). Coronal CT (c) shows the apposition of abnormal bone at the level of the fissula ante fenestram and round window
Precise documentation of the extent and location, in particular in regard to round window stenosis and cochlear lesions should be given in the report.
2.4.2 Other Bony Dysplasias
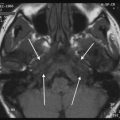
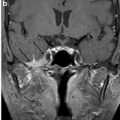
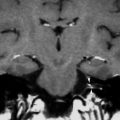
Bony disorders of the temporal bone such as fibrous dysplasia, Paget’s disease, osteopetrosis, or osteogenesis imperfecta may suffer from profound hearing loss. (Fig. 6) This is often due to encroachment of the external auditory canal, middle ear, and internal auditory canal. In Sickle cell disease, hearing loss is thought to be the result of vascular compromise of the cochlea.
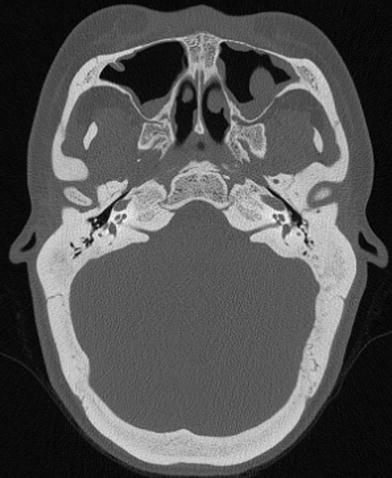

Fig. 6
Hearing loss due to bone dysplasia: in this patient with Robinow syndrome, a skeletal dysplasia characterized by dwarfism and craniofacial abnormalities, severe narrowing of the internal auditory canals is seen. The middle ear cavities were also encroached and the ossicles were thickened (not shown)
The value of cochlear implantation in these diseases is not yet clear.
2.5 Retrocochlear Disease
2.5.1 Cochleovestibular Nerve
Development of the cochleovestibular nerve is independent of the cochlea. If the cochlear nerve is absent, cochlear implantation is contraindicated. On CT, a narrow IAC (<2–3 mm) should raise the suspicion of cochlear nerve aplasia. Definitive diagnosis, however, can only be made with MRI.
Hypoplasia of the cochlear nerve has been correlated with poor outcomes of cochlear implantations. A strong positive correlation between the diameter of the cochlear, vestibular, and eighth cranial nerves with the total spiral ganglion cell has been reported (Nadol 1997), but the correlation between size and outcome still has to be established. Some have advocated the use of auditory brainstem implants (ABIs) in cochlear nerve hypoplasia (O’Driscoll 2011). In any case, the finding of cochlear nerve hypoplasia will influence patient counseling.
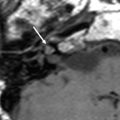
On CT, a small cochlear aperture may help to identify patients at risk for having a hypoplastic cochlear nerve (Fatterpekar et al. 2000) (Fig. 7). The nerve itself will be visualized on MR. Normally, the size of the cochlear nerve should be as large or larger than that of the facial nerve.
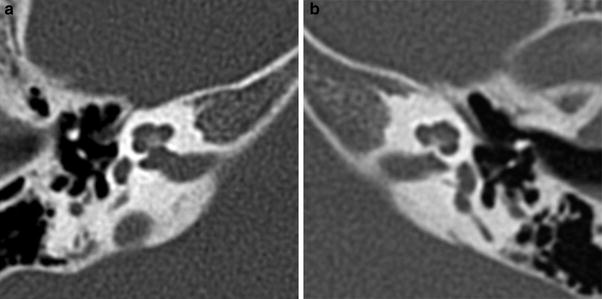

Fig. 7




Cochlear nerve hypo- or aplasia: in this child with unilateral congenital hearing loss on the left a normal width of the bony fenestration at the base of the modiolus is seen in the right ear (a), whereas on the left a stenosis of the cochlear aperture is present, suggestive of cochlear nerve aplasia
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree