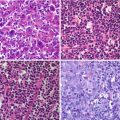
Fig. 23.1
Statistical parametric mapping (SPM) data in five HUS patients without neurological symptoms (paired data t test): acute vs. late PET studies. Volume-of-interest (VOI)-based analyses of PET data were performed by comparing serial PET studies; processing included the normalization of PET brain volumes to 3D templates (SPM). The normalized brain volumes were analyzed for regional specific FDG uptake, with SPM2 processing applied using the analysis of acute vs. recovery PET/CT studies. Hypometabolic regions are shown in blue and hypermetabolic regions in red
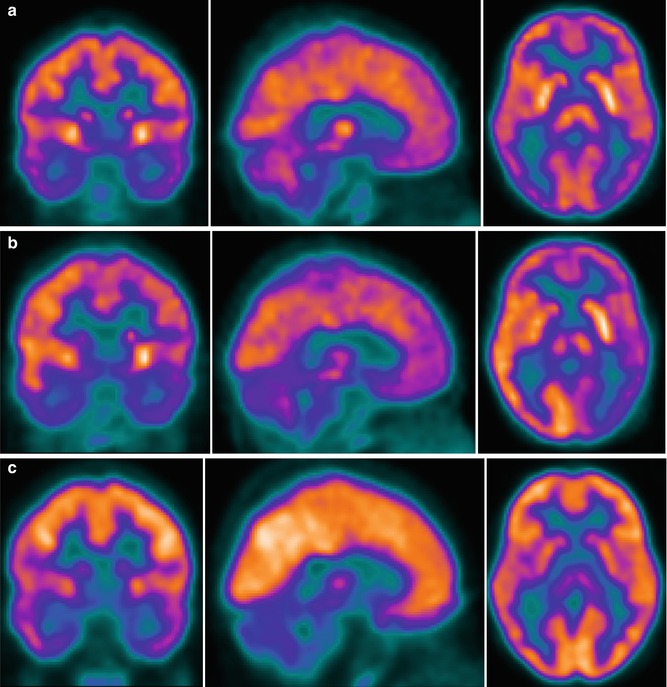
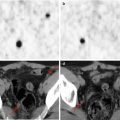
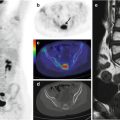
Fig. 23.2
PET/CT brain imaging in an 8-year-old boy. The 18F-FDG–PET study (Biograph TruePoint PET/CT, Siemens, Erlangen, Germany) was conducted 40–60 min after intravenous 18F-FDG administration (100–120 MBq). Low-dose CT sections of the head were used for attenuation correction. The 18F-FDG–PET study was performed on day 3, during acute HUS without neurological symptoms (a); on day 12, during acute HUS with neurological symptoms (b); and 3 months later, after clinical resolution (c)
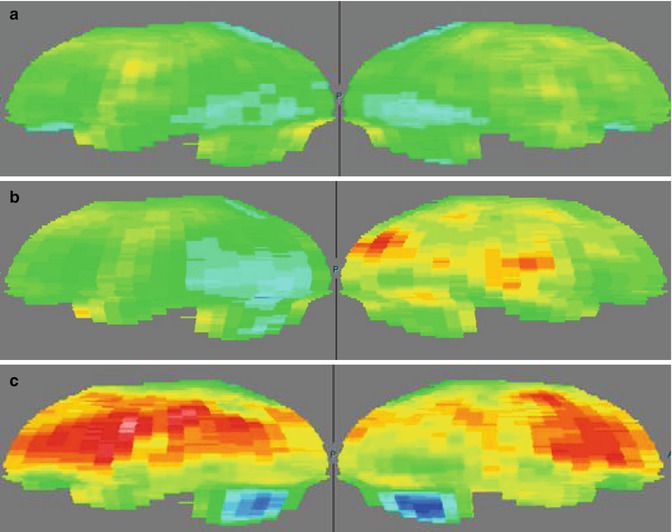
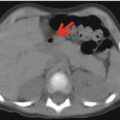
Fig. 23.3
18F-FDG–PET studies on day 3, during acute HUS without neurological symptoms (a); on day 12, during acute HUS with neurological symptoms (b); and 3 months later, after clinical resolution (c). The studies are compared with those in a healthy adult and were normalized to the Scenium brain volumetric template
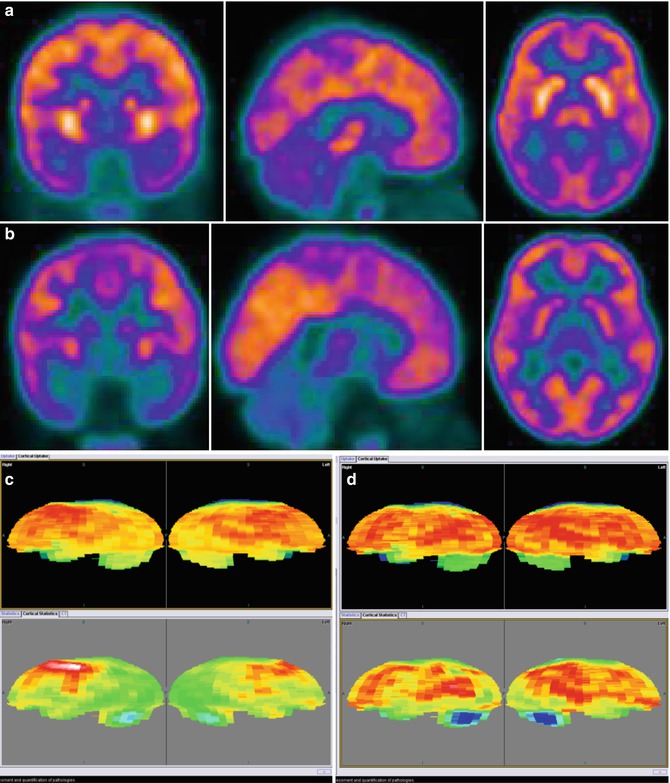
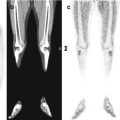
Fig. 23.4




18F-FDG–PET studies (Biograph TruePoint PET/CT tomograph, Siemens, Erlangen, Germany) in a 4-year-old girl during acute HUS (a) and 3 months later (b). (c, d) 18F-FDG–PET study in the same patient during acute HUS (c) and 3 months later, after clinical resolution (d), compared to the findings in a healthy adult and normalized to the Scenium™ brain volumetric template
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree