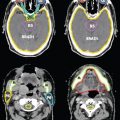
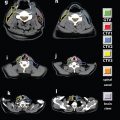
FIGURE 12-6. Illustration of a six-copolanar inverse IMRT whole-breast plan that minimizes dose to the heart.
• Breast cancer is the most common malignancy in women in the United States. The estimated number of new cases diagnosed in 2012 is 229,060. While incidence has been rising in the recent decades in United States, mortality has been declining with 17% of diagnosed patients dying of breast cancer in 2012.1
1. ANATOMY
• The mammary gland lies over the pectoralis major muscle and extends from the second to the sixth rib craniocaudally, and from the sternum to near the midaxillary line.
• The breast parenchyma is intermixed with connective tissue, which has a rich vascular and lymphatic network and is supported by fibrous septae known as Cooper ligaments. These septae connect the breast parenchyma to the overlying skin and the pectoralis fascia. These supporting structures can be affected by tumors and lead to skin dimpling.
• The predominant lymphatic drainage of the breast is to axillary lymph nodes. The axilla has three levels, based on the relationship of the lymph node regions to the pectoralis minor muscle. Level I axilla is caudal and lateral to the muscle, level II is beneath the muscle, and level III (also known as the infraclavicular region) is cranial and medial to the muscle. A standard axillary lymph node dissection resects the tissue and lymph nodes within levels I and II. The axillary lymph nodes continue underneath the clavicle to become the supraclavicular lymph nodes.
• Breast lymphatics can also drain directly into the internal mammary lymph node chain (IMC) usually limited to the first three interspaces. The internal mammary lymph nodes are intrathoracic structures located in the parasternal space. Breast cancers that develop in the medial, central, or lower breast more commonly drain to the IMC compared to breast cancer occurring in the lateral and upper quadrants (see Fig. 12-1).2
2. NATURAL HISTORY
2.1. Breast Cancer Development
• Breast cancer develops as a consequence of changes in epithelial cells and complex interactions between epithelial cells and the microenvironment. Some, but not all, breast cancers pass through a series of conditions ranging from atypical ductal hyperplasia (ADH), to noninvasive ductal carcinoma in situ (DCIS), and finally to invasive breast cancer. Many breast cancers develop in the absence of DCIS, suggesting that the ordered progression through the traditional biological spectrum is not required. Typical patterns of spread for breast cancer by T stage are illustrated in Figure 12-2.
• The progression rate of DCIS to invasive breast cancer is difficult to estimate and likely dependent on the grade of the lesion.
• Lobular carcinoma in situ (LCIS) has historically been felt to be more of a marker for breast cancer risk than a true premalignant condition for invasive breast cancer development, and the risk of ipsilateral and contralateral cancer is roughly equivalent.
• The kinetics of breast cancer evolution is heterogeneous, with some tumors being rather indolent, while others, such as inflammatory breast cancer, with rapid onset of symptoms and rapid progression.
• Risk factors for the development of breast cancer include
º Genetic: germline mutations in BRCA1, BRCA2, p53, PTEN
º Family History: positive family history of breast or ovarian cancer
º Estrogenic: nulliparity, late first pregnancy, early menarche, late menopause, postmenopausal estrogen/progesterone replacement
º Dietary: moderate or heavy alcohol use
º Pathology: personal history of prior breast cancer, DCIS, LICS, and ADH
2.2. Breast Cancer Progression
Invasive breast cancers can metastasize to regional lymph nodes (axillary, supraclavicular, and internal mammary nodal regions) or to distant sites at any point in their development history.
• Risk factors associated with increased incidence of spread to regional lymph nodes and distant sites include
º Primary tumor size (the risk of metastases is low for tumors under 1 cm in size)
º Primary tumor grade
º Presence of lymphovascular space invasion
º Nonfavorable histology (i.e., not tubular, medullary, or mucinous)
º Triple-negative tumors
3. STAGING SYSTEM
• All patients with breast cancer are staged using TNM staging system. In 2010, the American Joint Committee on Cancer implemented changes/additions of the TNM staging for breast cancer and generated the 7th edition of the TNM classification.3
• Pathological staging system is used for patients treated with surgery first, whereas for patients treated with chemotherapy before surgery (neoadjuvant chemotherapy) the clinical staging system is applied.
FIGURE 12-1. Lymphatic drainage of the right breast. (A) Most lymph drains to the axillary lymph nodes. (B) The red arrows indicate the direction of lymph flow from the axillary lymph nodes to the right lymphatic duct. (From Moore KL, Dalley AF. Clinically Oriented Anatomy, 4th ed. Baltimore, MD: Lippincott Williams & Wilkins, 1999.)
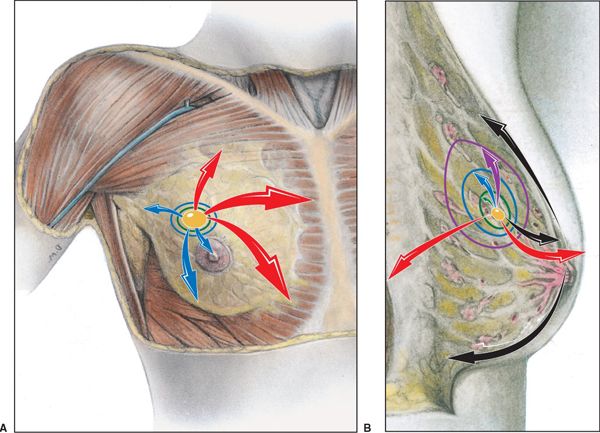
FIGURE 12-2. Patterns of spread for breast cancer. The primary cancer invades in various directions, which are color-coded vectors (arrows) representing stage of progression. Tis, yellow; T1, green; T2, blue; T3, purple; T4, red; and metastatic, black. (Reprinted from Rubin P, Hansen JT. TNM Staging Atlas with Oncoanatomy, 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2012:213. Modified from Agur AMR, Dalley AF, eds. Grant’s Atlas of Anatomy, 12th edition. Philadelphia: Lippincott Williams & Wilkins, 2009.)
3.1. Primary Tumor
Tumor stage is denoted as follows: Tis: DCIS or LCIS; T0: no evidence of primary tumor; T1: invasive disease up to 2 cm; T2: 2–5 cm; T3: larger than 5 cm; T4: tumor of any size with direct extension to the chest wall and/or the skin (ulceration or skin nodules), with subcategories: T4a: fixation to chest wall, T4b: involvement of skin, T4c: both, T4d: inflammatory breast cancer.
3.2. Regional Lymph Nodes (N)
Lymph node classification criteria differ depending on whether the nodes are clinically (cN) or pathologically (pN) assessed.
• Clinical N1 is described as suspicious mobile ipsilateral level I, II axillary lymph nodes; clinical N2 is defined as fixed or matted axillary lymph nodes or clinically detected ipsilateral internal mammary nodes (IMN) in the absence of clinically evident axillary node metastases; clinical N3 includes infraclavicular or supraclavicular lymphadenopathy or clinically detected ipsilateral internal mammary lymph node(s) with clinically evident level I, II axillary lymph node metastases.
• Pathologic classification of regional lymph nodes include pN0 defined as no histologic regional lymph node metastasis; pN1, micrometastases, or metastases in one to three axillary lymph nodes; pN2, metastases in four to nine axillary lymph nodes; pN3, metastases in 10 or more axillary lymph nodes; or in infraclavicular (level III axillary) lymph nodes.
º Patients are considered to have M1 (stage IV) breast cancer if metastases were detectable before neoadjuvant therapy, regardless of their status after neoadjuvant treatment. M0 is defined as absence of distant metastases based on clinical or radiographic evidence: cM0(i+) when no clinical or radiographic evidence of distant metastases is present, but deposits of molecularly or microscopically detected tumor cells are found in circulating blood, bone marrow, or other nonregional nodal tissue that are no larger than 0.2 mm in a patient without symptoms or signs of metastases; M1 is defined as distant detectable metastases determined by clinical and radiographic means or histologically proven larger than 0.2 mm.
º An important change made to the T, N, and M categories in the 7th edition of the staging system for breast cancer involves the definition of ypTNM nomenclature used for cases where systemic and/or radiation therapy are given before surgery (neoadjuvant setting). The posttreatment ypT is the largest contiguous focus of invasive cancer as defined histopathologically. Posttreatment nodal metastases no greater than 0.2 mm are classified as ypN0(+).
4. CLINICAL PRESENTATION AND DIAGNOSTIC WORKUP
• Most patients with carcinoma in situ, T1, and T2 breast cancers present with an abnormal screening mammogram or have a painless or slightly tender palpable breast mass.
• Locally advanced breast cancer can arise from a tumor that has been present for a long period of time which grows within the breast and spreads to nearby areas including lymph nodes, skin, and muscles or can present as a rapidly growing breast mass with or without redness, swelling, and peau d’orange. The latter is commonly referred to as inflammatory breast cancer.
• Metastases can occur through lymphatic or hematogenous spread; the former involves the axillary, internal mammary or supraclavicular nodes and the latter causes spread to bone, lung, pleura, liver, and brain.
• All patients should undergo workup that includes
º History and physical exam
º Diagnostic bilateral mammogram and ultrasound. Ultrasonography, which has a sensitivity of 73% and specificity of 95%, is helpful in differentiating cysts from solid tumors.
º Tissue diagnosis: Biopsy of the palpable breast lesion and palpable regional adenopathy and nonpalpable lesions detected on imaging studies and needle localization with radiographic techniques.
º Estrogen (ER) and progesterone receptor (PR) status on both invasive and noninvasive breast cancers as well as assay to assess overexpression of Her-2Neu is obtained.
º Breast magnetic resonance imaging (MRI) is optional and can be done when the breast is mammographically dense to evaluate extent of disease in the presence of multifocal or multicentric disease and for diagnosis of questionable findings seen on physical examination, mammography, or ultrasound.
º CBC, serum chemistry profile including liver function tests and alkaline phosphatase.
• In patients with stage 0, I, or II disease, the incidence of abnormal bone scan is approximately 2% and it is not routinely obtained unless there is localized bone pain or elevated alkaline phosphatase.
• For more advanced tumors, computed tomography (CT) scans of chest, abdomen, and bone will diagnose or rule out distant metastases. Alternatively, a positron emission tomography (PET)/CT scan can be obtained, but is considered optional at this time.1
• If neurologic symptoms suggest cerebral metastases, a contrast-enhanced CT scan or, for better diagnosis, gadolinium-enhanced MRI scan of the brain should be obtained.
5. MANAGEMENT
• The management of breast cancer depends on prognostic factors including stage, histologic type, nuclear and architectural grade, differentiation, tumor hormone receptor content (ER, PR, HER2-Neu receptors), Ki-67, presence of lympho-vascular invasion, menopausal status at the time of diagnosis, and comorbid conditions.
• Management usually consists of a combination of local-regional surgery, radiotherapy (RT), and systemic treatment including cytotoxic chemotherapy, endocrine therapy, biologic agents.
• The following chapter will focus on local-regional management with RT and the application of intensity-modulated radiotherapy (IMRT).
5.1. Local-Regional Management of Stage 0, I, and II Breast Cancer
5.1.1. Stage 0 (TisN0M0)
• Mastectomy and lumpectomy with negative margins followed by breast RT offer equal overall and cause-specific survival and represent options for treatment of pure DCIS. This equivalence has been demonstrated by four randomized trials with long follow-up of 10–20 years. If tumor cells are positive for ER, hormonal therapy is added for additional benefit in decreasing ipsilateral breast recurrence as well as contralateral breast cancer diagnosis.
• RT administered after lumpectomy decreases invasive and noninvasive recurrences by 50% to 60% in the treated breast.
5.1.2. Early Stage—I and II Breast Cancer (T1,T2/N0, N1mic, N1/M0; T3 N0 M0)
• Treatment options for early breast cancer include mastectomy with sentinel node biopsy/axillary node dissection or breast conserving therapy (BCT) consisting of lumpectomy, sentinel lymph node biopsy/axillary node dissection followed by breast irradiation.
• Six randomized trials with mature data of 10 to more than 20 years follow-up revealed equivalence between mastectomy and BCT regarding cause-specific survival and overall survival. Clinical trials also showed that RT added to lumpectomy reduces tumor recurrence in the treated breast by two-thirds compared to lumpectomy alone, with no effect on overall survival by individual trials.
• The most recent Early Breast Cancer Trialist’s Group (EBCTG) individual patient meta-analysis showed that adding RT to breast conserving surgery significantly reduces the breast cancer–specific mortality by 3.8% for pN0 patients and 8.5% for pN+ patients with an overall improvement in survival of 4.4% at 15 years.4
• With well-established benefit, radiation is a recommended component of BCT.
• Chemotherapy and/or hormonal therapy are incorporated into the management in selected patients. RT usually follows chemotherapy completion, whereas hormonal therapy is in general started after the course of RT is finished.
5.1.3. Radiation Therapy Doses for Whole-Breast Radiation
• Traditionally, in postoperative setting RT has been administered to the entire breast in daily fractions for 5 weeks followed by additional boost dose to the tumor bed for 1–1.5 weeks.
• In general, radiation doses to the entire breast consist of 45–50 Gy in 1.8–2.0 Gy/fraction, 5 days/week. Photons with energies of 4 to 6 MV (<10 MV) are preferred to treat the breast. Wedges (dynamic or static) or compensating filters have been used to achieve a uniform dose distribution within the breast (ideally <7% dose variance across the target volume).
• The boost to the tumor bed, when necessary, can be administered using electrons or photon beams for additional 10–16 Gy in 2 Gy fractions, 5 days/week.
• In recent years, shorter radiation courses of RT have been investigated. Treating the entire breast with hypofractionated accelerated schemes was found in selected patients to be comparable with traditional 6–7 weeks regimen in achieving local control, cosmetic results, and survival.
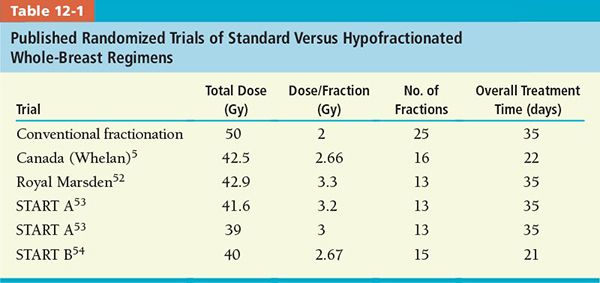
• A common fractionation scheme applied to the whole breast accelerated treatment is whole breast dose of 42.5 Gy in 2.66 Gy/fraction for 16 days treatment.5
• Table 12-1 illustrates published randomized trials of standard versus hypofractionated whole-breast regimens. Tumor bed boost was not administered in the Canadian (Wheelan) protocol, while the other protocols were left at the discretion of treating physician.
• An American Society for Therapeutic Radiation Oncology (ASTRO) task force performed a systematic literature review of hypofractionated whole-breast RT.6
• A set of criteria was generated for short RT course eligibility as following:
º age 50 years or older at diagnosis
º pathological stage T1–T2 N0
º no systemic chemotherapy administration
º dose variation along the central axis of 93% to 107%
5.2. Accelerated Partial-Breast Irradiation
• To further shorten the course of RT, the lumpectomy cavity only can be treated using large dose per fraction administered to smaller volumes with acceptable toxicity. The rationale of this approach is the observation that most recurrences occur in the same quadrant with initial location of the tumor.
• A panel of experts selected by ASTRO generated a consensus of patient eligibility for partial-breast RT administration (see Table 12-2) based on review of 4 randomized trials and 38 retrospective studies.7 Single-arm, single-institution published studies revealed that selected, low-risk patients treated with accelerated partial-breast irradiation (APBI) had a breast cancer recurrence of 3% to 5% at 2–8 years follow-up.
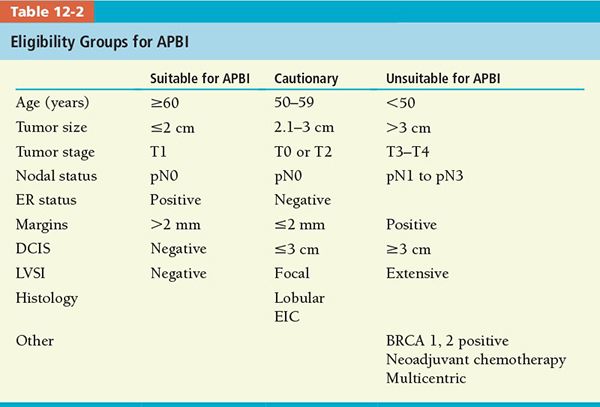
• Although a variety of fractionated schemes have been studied, the most frequent partial-breast radiation dose used outside a clinical trial, administered using either mammosite balloon or external beam RT, is 38.5 Gy in 10 fractions (3.85 Gy per fraction), 2 fractions per day, with interfraction interval of at least 6 hours. At the time this chapter was written, there were three open phase III, randomized trials that compared whole-breast radiation therapy with APBI (see Table 12-3).
• Until results of these randomized trials are available, ASTRO consensus statement of selection criteria for APBI should be applied outside of clinical trials.
5.3. Radiation Therapy Techniques for Treatment of the Whole Breast
• Early planning of radiation treatment used two-dimensional (2D) approach, with isodose distribution being visualized in only one plane (central axis). CT scan–based simulation brought improvement in treatment planning allowing visualization and adjustments of dose distribution through the entire breast. This has implications in clinical outcome as has been demonstrated that dose homogeneity within the breast and technical methods to improve it influence clinical outcomes of toxicity and cosmesis.
5.3.1. Treatment Volume
• With patient in supine position, the tangent fields have the following borders:
º The upper margin is placed at the head of the clavicle.
º The lateral/posterior margin is placed 2 cm beyond palpable breast tissue (usually at the midaxillary line or anterior extend of the latissimus dorsi muscle that can be visualized on CT slices).
º The inferior margin is placed 2 cm below the inframammary fold or below the mammary gland for pendulous breast.
º The medial margin is placed at the anterior midline chest.
• Other treatment positions have been introduced in practice to improve dosimetry in patients with large, pendulous breasts, like lateral decubitus position to flatten the breast contour.
• More popular technique is treatment in prone position on a special prone breast board with the treated breast positioned vertically to gravity, away from the chest wall.
• Figure 12-3 illustrates tangent treatment fields projected on patient’s body with borders as described.
5.3.2. IMRT for Breast Treatment
• Improvement in therapeutic ratio of breast irradiation has been achieved with CT-based, three-dimensional (3D) planning methods. IMRT offers additional potential dosimetric benefit in maximizing target coverage and tumor control, while minimizing toxicity to the treated breast and surrounding normal structures.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree