Breast Ultrasound
EQUIPMENT AND TECHNICAL ISSUES
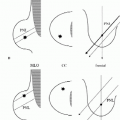
Spatial resolution in ultrasound is defined as axial, lateral, and elevation resolution (Figure 4.1). Axial resolution is the ability to resolve two adjacent structures along the axis of the beam and is determined by the pulse length (wavelength times number of cycles per pulse). Pulse length decreases as the frequency of the transducer increases. Consequently, high-frequency transducers have greater axial resolution; however, the higher frequencies are attenuated more quickly. These characteristics make high-frequency transducers the choice for imaging breast tissue when maximal axial resolution is desired in relatively thin tissue. Determined by the width of the ultrasound beam, lateral resolution is the ability to resolve adjacent structures that are perpendicular to the beam, parallel to the long axis of the transducer. Focusing of the beam can alter the beam width at selected depths of interest. Elevation resolution is the ability to resolve adjacent structures in a plane perpendicular to the beam and long axis of the transducer. The thickness of the ultrasound beam, a factor not controlled by the operator, determines elevation resolution.
Axial resolution is greater for high-frequency transducers, making them optimal for imaging breast tissue.
Lateral resolution is determined by the width of the beam and can be altered by focusing the ultrasound beam.
Contrast resolution refers to the ability of an ultrasound unit to resolve two objects with different but similar echogenicities; it is directly affected by the dynamic range setting on the ultrasound unit. When the dynamic range setting is too low, image contrast is increased, such that solid masses may appear cystic. Conversely, if the dynamic range is too high, there is little contrast in the image, and subtle solid masses are rendered indistinguishable from adjacent fat lobules (1).
The dynamic range setting on the ultrasound unit directly affects the ability to resolve objects with similar but different echogenicities (contrast resolution).
Our dedicated ultrasound equipment is in immediate proximity to the mammography and stereotactic equipment. The units are equipped with 5- to 10-MHz and 6- to 13-MHz transducers. Doppler and tissue harmonic imaging are available on both units. The 6- to 13-MHz transducer is the most commonly used. However, the 5- to 10-MHz transducer is helpful in evaluating patients with larger breasts and lesions that are deep in the breast. This transducer is also helpful in women with dense tissue in whom we see significant amounts of artifactual shadowing when scanning with the higher-frequency transducer.
Optimization of the scanning parameters is critical. As discussed with mammographic image quality, it is important that you maximize ultrasound image quality. Set your quality standards high, and monitor image quality on a patient-by-patient basis. Do not settle for suboptimal equipment or images. Review and become familiar with the guidelines from the American College of Radiology (ACR) regarding breast ultrasound (2). Unfortunately, and somewhat reminiscent of the experience with mammography image quality in the 1980s and early 1990s, breast ultrasound image quality at many facilities may not be what is required for good diagnostic work. On a review of 152 breast ultrasound examinations from 86 different institutions, Baker and Soo reported noncompliance with at least
one of the ACR guidelines for breast ultrasound in 60.5% of studies (3). It is only when optimized equipment, image quality, and meticulous technique are used that ultrasound becomes an invaluable tool in the evaluation and management of women with breast-related diseases. Anything else is often misleading and may be worse than nothing.
one of the ACR guidelines for breast ultrasound in 60.5% of studies (3). It is only when optimized equipment, image quality, and meticulous technique are used that ultrasound becomes an invaluable tool in the evaluation and management of women with breast-related diseases. Anything else is often misleading and may be worse than nothing.
Optimized equipment, high image quality, and meticulous technique are critical for diagnostic ultrasound work in breast imaging. Set your quality standards high, and monitor image quality on an ongoing basis.
The number and positioning of the focal zones need to be set appropriately. The focal zones should be at the level of the lesion or, at most, 1 cm superficial or deep to the anterior and posterior margins of the mass, respectively. Gain settings should enable the distinction between a simple cyst and solid mass. In the absence of a cyst, fat lobules should have varying shades of gray and not be so light as to be white or so dark as to be black. The field of view is adjusted so that an adequate amount of tissue is seen surrounding the lesion being evaluated. It should be possible to establish the distance from the skin to the mass, and there should be enough posterior tissue to assess any changes in the transmission of the ultrasound beam beyond or deep to the lesion (3). In patients with large masses, it may be difficult to image the entire lesion and surrounding tissue. With adjustments in technique as needed, evaluation of the lesion in segments may be indicated. Depending on the unit and transducer used, a standoff pad may be needed to evaluate superficial lesions. The standoff pad increases the distance from the transducer to the lesion, helping position the lesion at an appropriate depth of focus for the transducer. If the standoff pad is not used for superficial lesions, near-field artifacts may be created, leading to an inaccurate characterization of lesions.
Some minimal considerations when performing breast ultrasound examinations are listed in Box 4.1.
Box 4.1: Breast Ultrasound: Minimal Considerations
Greater than 7 MHz, linear array transducer (center frequency at 6 MHz for broadband transducers)
Focal zone positioning
Gain setting
Field of view
Lesion in perpendicular projections
Maximal lesion dimensions
Label images: laterality, lesion location (o’clock position, quadrant or shown on diagram of breast), probe orientation
Label films: patient’s first and last name, unique identification number, facility name, location, examination name, and operator’s identification
The use of film to display and store images from breast ultrasound studies is recommended. Some facilities print their images on strips of paper. For appropriate preservation of the images, quality paper and appropriate storage need to be used; otherwise, the images fade. Also, it is best if paper images are secured in plastic sleeves; otherwise, they can become mangled as the contents of the patient’s film jacket are manipulated. If you are working in a Picture, Archiving and Comment section System (PACS) environment, these issues become secondary.
SCANNING TECHNIQUE
Breast ultrasound is an adjunctive method used to evaluate areas of mammographic or clinical concern. Although the entire breast can be scanned, studies limited to the area of mammographic or clinical concern are more common. The patient is positioned so that the tissue in the area of interest is thinned as much as possible. Supine positioning is used when evaluating the inner quadrants; decubitus positioning is used for far lateral tissue, and oblique positioning for tissue in the upper outer quadrants. In evaluating the lateral quadrants, having the patient raise the ipsilateral arm and place it comfortably under her head helps in further thinning the area to be scanned. One of the advantages of ultrasound is its real-time capabilities. Patients are positioned as needed to evaluate the area of concern. If the patient describes an abnormality as more apparent in the upright position, she is scanned upright. When there are significant physical limitations, however, patients can be scanned in a wheelchair or stretcher.
Position the patient so that the area being evaluated is thinned as much as possible.
The reason for the study and how the study is done are explained to the patient. The coupling gel is prewarmed in commercially available warmers. If the gel is used at room temperature, it can be uncomfortably cold, particularly in the winter months. As with positioning for the mammographic study, it is important to consider the patient’s modesty. Only the breast to be examined and scanned is exposed. The contralateral breast is kept covered unless a comparison needs to be done. At the completion of the study, the patient is provided a towel to wipe the coupling gel off her breast. A second staff person is always in the room when we scan patients.
Scanning by the radiologist is encouraged strongly. The breast imaging radiologists do all of the breast ultrasounds at our facility. We do not use an ultrasound technologist. A mammography technologist, or an assistant, helps us during diagnostic and interventional ultrasounds but does not perform the scan. We have several reasons for taking this approach. First, by having the radiologist do the ultrasound studies, we are able to examine the patient and correlate what is described or seen on the mammogram with our own physical exam. Real-time scanning and palpation are critical to our final impression. Second, ultrasound is an operator-dependent study. Normal tissue can be made to simulate a lesion, and true lesions can be easily overlooked. To establish the presence of a lesion, the transducer needs to be rotated over the potential lesion, using variable amounts of pressure to eliminate artifactual shadowing generated by ligaments. Third, as the physical examination is done, and an impression is generated during real-time scanning, selective images are taken to document the features of the lesion, leading us to make a specific recommendation (Table 4.1). We do not take images of normal tissue. Finally, by doing the ultrasound ourselves, we can establish rapport with patients. We can elicit pertinent history, reassure the patient that we are doing everything possible to take care of her, and discuss findings and recommendations. The patient’s input is sought, and she is involved in the decision-making process. After discussing all reasonable alternatives, we provide the patient with a specific recommendation.
Correlative physical examination is done as the patient is scanned. The ultrasound coupling gel improves perception of palpable abnormalities. The transducer is moved in small increments over an area, while the index, middle, and ring fingers of the contralateral hand are placed at the leading edge of the transducer and used to palpate the area being scanned. If a possible abnormality is detected, the transducer is rotated 360 degrees over the area to help distinguish a mass from a fat lobule in cross-section. A mass maintains
a round, oval, or irregular shape as the transducer is rotated, whereas fatty tissue elongates and fuses with surrounding structures. Traditionally, transverse and longitudinal orientations are used for imaging, but because ductal structures radiate out from the nipple toward the chest wall, radial and antiradial scan orientations are recommended by some.
a round, oval, or irregular shape as the transducer is rotated, whereas fatty tissue elongates and fuses with surrounding structures. Traditionally, transverse and longitudinal orientations are used for imaging, but because ductal structures radiate out from the nipple toward the chest wall, radial and antiradial scan orientations are recommended by some.
As you are doing the study, undertake correlative physical examination by placing the pads of the index, middle, and ring fingers (dominant hand) at the leading edge of the transducer. This is the equivalent of having eyeballs on the tips of your fingers.
Table 4.1: Breast Ultrasound: Features of Lesions | ||||||
|---|---|---|---|---|---|---|
|
Images are not taken until a decision is made about the presence of a lesion. Making this determination involves correlation with mammographic and clinical findings, slow movements and rotations of the transducer over the area of concern, gradations in compression, time gain compensation (TGC) curve and power-setting manipulations, positioning of the focal zones, correct field of view, and if indicated, use of a standoff pad. If during real-time scanning it is determined that a lesion is present, images are then taken to document the presence and features of the lesion. Using whatever orientations demonstrate the features of the mass best, orthogonal images are taken with and without measurements for a total of four images per lesion. The images are annotated (2,3) to include the name of the patient, unique patient identifying number, date of study, name of facility, breast being imaged, o’clock location of the lesion (Figure 4.2A), distance of the lesion from the nipple, transducer orientation (radial/antiradial; transverse/longitudinal) (Figure 4.2B) and depth in the breast (retroareolar, anterior third, middle portion, posterior third, axillary tail, axilla). As the transducer is manipulated, the size of the lesion varies. In providing a measurement, an attempt is made to use the largest dimensions of the lesion (2,3). It is also useful to indicate on the film whether the lesion is palpable.
TERMINOLOGY
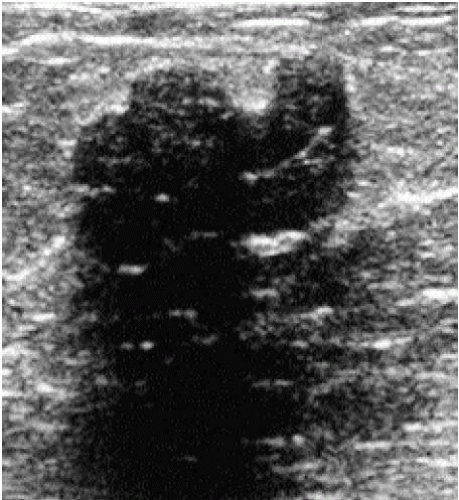
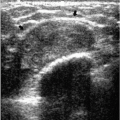
Several terms are used to characterize the appearance of tissue and lesions on ultrasound. In the breast, the echogenicity of an area of interest is compared to the appearance of subcutaneous fat. If the area contains fewer echoes (i.e., appears darker) compared with subcutaneous fat, it is hypoechoic; if it contains more echoes (i.e., appears whiter), it is hyperechoic; if it contains no echoes (i.e., it is black), it is anechoic; and if it is of the same echogenicity as subcutaneous fat, it is isoechoic. At the junction of tissues with significant acoustic impedance differences, variable amounts of the sound beam are reflected back, and a shadow is seen. The size of the shadow depends on the size of the interface. Shadowing is a feature of some lesions, and although more common with malignant lesions, it is also seen with some benign masses (Figure 4.3). In lesions associated with calcifications, shadowing is sometimes seen in association with the calcifications (Figure 4.4).
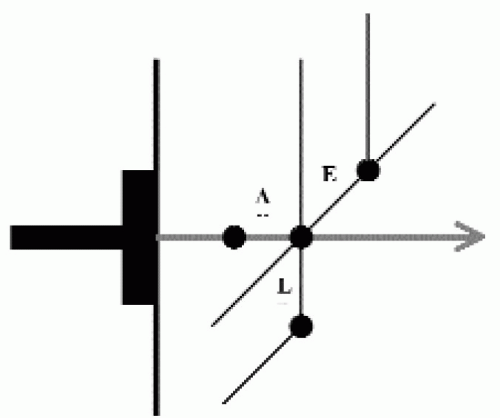
 Figure 4.2 Image labeling, ultrasound. A. Lesion location is indicated by using the o’clock position of the lesion in the breast and the distance of the lesion from the nipple. B. Images are obtained in orthogonal projections. This includes radial and antiradial (1) or transverse and longitudinal (2). |
Simple cysts do not attenuate sound energy. Consequently, a proportionately greater amount of sound energy reaches the tissues deep to a cyst. The TGC curve, however, is compensating for presumed decreases in echo strength as the distance from the transducer increases. The resulting effect is that tissue deep to cysts appears more echogenic than adjacent tissue. This increased echogenicity deep to fluid-filled structures is called posterior acoustic enhancement or increased sound (through) transmission. Enhancement is common with cysts; however, it can be seen with benign (Figure 4.4) and malignant solid masses.
BREAST ANATOMY ON ULTRASOUND
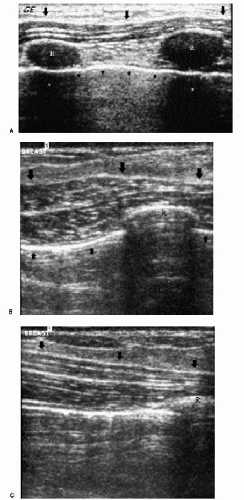
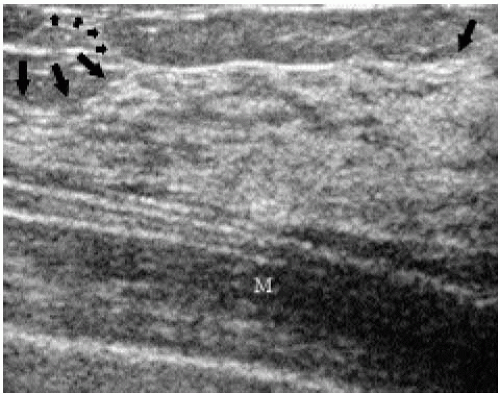
Understanding the anatomy of the breast on ultrasound is helpful in evaluating potential lesions. The pleural reflection, ribs, and pectoral muscles are deep to breast tissue and usually imaged during scanning. The pleural reflection is a hyperechoic band deep to the ribs. The tissue-air interface usually leads to shadowing deep to the pleural reflection. Simulating lesions, ribs in cross-section are well-circumscribed, oval structures (Figure 4.5A). Recognizing the relationship of the ribs to the pleural reflection and overlying pectoral muscles is important in distinguishing a rib in cross-section from a lesion. Longitudinally, ribs are echogenic bands of variable thickness with shadowing (Figure 4.5B). The pectoral muscles are hypoechoic with associated specular echoes that may be seen as bright spots in the muscle when in cross-section or as echogenic, parallel hyperechoic bands when imaged longitudinally (Figure 4.5C). The deep pectoral fascia is an echogenic line on the surface of the pectoral muscle that separates the muscle from overlying breast tissue.
Rotation of the transducer (90 degrees) over the area being evaluated during real-time scanning is critical in characterizing lesions and distinguishing them from bundles of normal tissue.
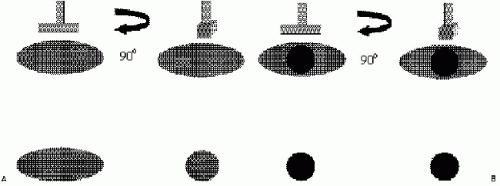
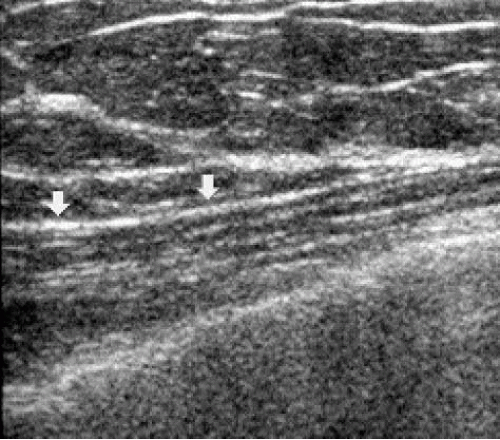
Cooper’s ligaments connect deep and superficial pectoral fascial layers, thereby providing a honeycomb-like structure or a “skeleton” for breast tissue. The ligaments are hyperechoic bands that crisscross the breast isolating oval and oblong areas of glandular and fatty tissues. Superficially, Cooper’s ligaments extend to the superficial pectoral fascia in the deep dermis. Given the oval and oblong shape of breast tissue bundles, transducer movements and rotation over an area of concern are important. When breast tissue bundles are imaged in cross-section as round or oval structures, they can sometimes simulate a lesion. As the transducer is rotated 90 degrees, breast tissue bundles elongate and fuse with surrounding tissue; a lesion will maintain its round or oval shape (Figure 4.6). Breast tissue bundles also commonly have small, hyperechoic bands within them not typically seen in solid masses (Figure 4.7).
Dense glandular tissue is relatively hyperechoic (Figure 4.8). Because most breast lesions are hypoechoic relative to subcutaneous fat, lesions are identified more readily in women with dense tissue mammographically. Cooper’s ligaments are not as apparent in hyperechoic fibrous tissue because they are isoechoic with fibrous tissue. In women with predominantly fatty tissue, however, lesions may be isoechoic with surrounding tissue and not identifiable. In fatty tissue, Cooper’s ligaments are identified more easily because they are hyperechoic relative to the surrounding tissue (Figure 4.9). In this patient population, however, mammography is reliable in depicting small water-density lesions. During pregnancy, breast tissue usually becomes more homogeneous with small round and oval anechoic spaces. Cooper’s ligaments, fibrous ridges, and tissue bundles are not as readily apparent (Figure 4.10). Prominent ductal structures may be seen, particularly in the subareolar area.
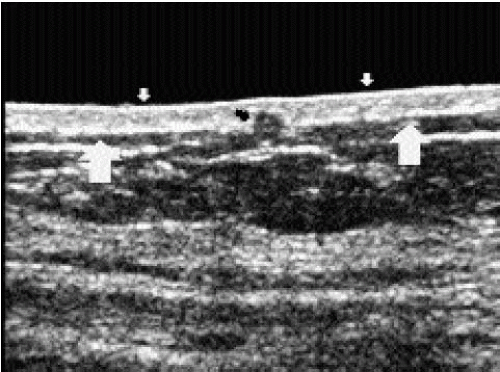
Use of the standoff pad is often required for evaluation of the skin. Normally, skin measures 1 to 2 mm and is made up of a hypoechoic band sandwiched between two hyperechoic lines (Figure 4.11). Edema resulting from radiation therapy, congestive heart failure, benign inflammatory processes, or inflammatory carcinoma can present with skin thickening. The deep hyperechoic line may be disrupted, and there is thickening of the hypoechoic band. Small, anastomosing, serpiginous tubular structures, representing
dilated lymphatics, are sometimes seen deep to the thickened skin (Figure 4.12). Malignant and inflammatory processes arising in breast tissue can extend to involve the skin, with disruption of the deep hyperechoic strip and expansion in the hypoechoic band. Benign lesions (e.g., sebaceous cysts) involving the skin, and sometimes arising in the hypoechoic band, can be localized, and their connection to the skin surface through pores can sometimes be identified. Rarely, metastatic disease to the skin can be imaged as thickening of the hypoechoic band with disruption of the superficial echogenic line when there is associated skin ulceration (Figure 4.13).
dilated lymphatics, are sometimes seen deep to the thickened skin (Figure 4.12). Malignant and inflammatory processes arising in breast tissue can extend to involve the skin, with disruption of the deep hyperechoic strip and expansion in the hypoechoic band. Benign lesions (e.g., sebaceous cysts) involving the skin, and sometimes arising in the hypoechoic band, can be localized, and their connection to the skin surface through pores can sometimes be identified. Rarely, metastatic disease to the skin can be imaged as thickening of the hypoechoic band with disruption of the superficial echogenic line when there is associated skin ulceration (Figure 4.13).
The nipple contains connective tissue and smooth muscle. In some women, it can simulate a mass; in others, it produces intense shadowing (Figure 4.14A, B). To evaluate the subareolar area, the transducer needs to be angled around the nipple. Ductal structures can be seen in the subareolar area coursing toward the nipple (Figure 4.14C, D). Intraductal lesions, such as papillomas, can sometimes be identified, particularly if the lesion is in the subareolar portion of the duct (Figure 4.15). Radial scanning is helpful in demonstrating the ducts coursing for variable distances away from the nipple.
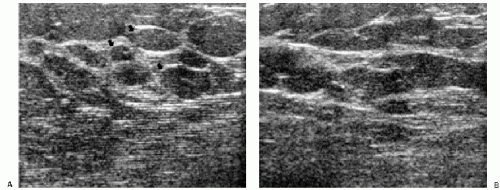
 Figure 4.12 Skin thickening and dilated subcutaneous lymphatics in a patient with congestive heart failure. A. Skin thickening is noted in this patient with congestive heart failure. The superficial and deep echogenic lines are not apparent, and the hypoechoic band is thickened. Tubular, anastomosing, serpiginous structures deep to the skin are dilated lymphatics. The tissue is echogenic, and normal tissue planes are disrupted. B. Same patient, different area of the breast. In this area, thin lymphatic channels can be seen in the hypoechoic band of the skin (arrows).
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|