Fig. 1
Scheme of particle distribution in tissues. Shown are several electrons and an alpha particle. Clearly, total energy absorbed per unit mass, i.e., dose correlates with the number of particles in that mass
The microdose values compose a spectrum according to charged particle energies from a given radiation quality. This spectrum may vary by a factor of up to ten or more, around the mean value. According to the radiation quality, the mean microdoses have defined values, as shown in Table 1 middle column. In case of exposure from incorporated radionulcides, the overlaying and more or less severe topographical heterogeneity of decays occurring localized in the tissue of interest makes dosimetry more difficult and has been dealt with extensively (ICRU 2002).
Table 1
The energy absorbed per micromass, here of 1 ng, per particle traversal is formally called specific energy with the symbol z, and 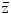 F1 is the fluency-derived mean value of z (33). The table gives the value of
F1 is the fluency-derived mean value of z (33). The table gives the value of 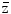 F1 and the approximate number of reactive oxygen species (ROS) produced by this value in the hit micromass
F1 and the approximate number of reactive oxygen species (ROS) produced by this value in the hit micromass
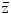 F1 is the fluency-derived mean value of z (33). The table gives the value of
F1 is the fluency-derived mean value of z (33). The table gives the value of 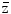 F1 and the approximate number of reactive oxygen species (ROS) produced by this value in the hit micromass
F1 and the approximate number of reactive oxygen species (ROS) produced by this value in the hit micromass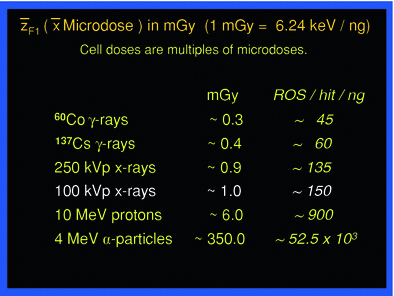 |
In the context of comparing man-made low-level exposures, for instance in diagnostic medicine, with exposure from natural sources of background radiation the following considerations may be helpful in risk assessment. As an example, the exposure of tissue to 100 kVp X-rays causes on average 1 electron track delivering about 6 keV per 1 ng mass—corresponding to the average cell mass—and the mean microdose is about 1 mGy (ICRU 1983). A body dose of 1 mGy from 100 kVp X-rays then means an average of about 1 microdose event in each ng mass of the exposed tissue. One mGy per year accordingly means that about 1 event per ng occurs per year, or each ng experiences on average one microdose of 1 mGy once about every 365 days.
Normal background radiation causes whole-body absorbed doses in the range of several mSv per year from different radiation sources and qualities, largely cosmic gamma rays with a relatively small contribution from alpha irradiation coming mainly from inhaled radon. Background radiation may vary considerably with altitude and geographic region, and may be more than 10-fold higher than the average value at sea level in the northern hemisphere. The above considerations imply that every ng or cell in the body experiences a microdose event several times a year. More specifically, taking an adult body to have 7 × 1013 ng, and a year to have about 3.2 × 107 s, then, for the sake of easy calculation, an annual whole-body dose of 1 mGy from chronic exposure to X-rays causes per second around 2.2 × 106 ng to have 1 mGy-microdose event on average, and each of those events would have the potential of triggering secondary consequences.
Such calculations are easy also for other radiation qualities than 100 kVp X-rays. The mean microdose values are displayed for a few different types of radiation in Table 1. Thus, if the background radiation field would be equivalent to gamma rays from 137Cs delivering about 0.4 mGy per microdose event, chronic exposure to an annual whole-body dose of 2 mGy would cause on average 1 microdose event five times a year in each ng in the body, or each ng would experience on average one event every 2.4 months.
The microdosimetry approach used here to describe energy deposition in exposed tissue helps to understand mechanistically what happens at low doses and dose-rates physically and biologically. This approach is consistent also with the International Commission on Radiation Units and Measurement (ICRU 2011).
3 Primary Biological Interactions
Each microdose event whether from external sources or from internally deposited radionuclides causes numerous atomic ionizations and excitations stochastically along the particle track depending on the type of radiation. Biological tissues consist by weight of ~75 % water. Hence, a correspondingly large fraction of ionizations induce hydrolysis resulting in reactive oxygen species (ROS); on average about 25 ROS are produced by hydrolysis for each keV absorbed in tissue, based on the expenditure of an average of 30 eV per ionization. The number of ROS per each mean microdose event from different radiation qualities is also listed in Table 1, right column. In general, ROS are both signaling molecules and can be toxic (Sen et al. 2000) depending on concentration. When produced by irradiation ROS attack largely at random all kinds of biochemical substrates in the immediate and in some distant molecular “neighborhood” of the site of their creation, and add to biochemical damage by direct ionizations.
The ROS induced by radiation are biochemically similar to those that are constantly and abundantly produced in different cellular compartments, mainly mitochondria, during normal oxidative metabolism. Mitochondria alone let leak out some 109 ROS into the cytosol per cell per day (Pollycove and Feinendegen 2003). One needs to consider the effects of both endogenous and radiogenic ROS alongside with direct effects, especially on DNA. The latter effects generally are more toxic but less frequent than the first.
4 Damage to DNA and its Repair
Biological responses to ionizing radiation, wherever they become observable either acute or delayed, appear to always originate because of changes in cellular molecules, especially the DNA. The immediate DNA damage includes inter-molecular cross links of various kinds, base changes, single-strand breaks (SSB), and the more serious double-strand breaks (DSB) (Hall and Giaccia 2005).
It needs to be stressed that the radiation-induced immediate damages to DNA increase linearly with dose. The reason for this linearity is the dose-dependent number of microdose events produced, and each of them causes a given degree of damage according to their energy spectrum characteristic for a given type of radiation. Thus, as dose increases, the number of microdose events according to the given spectrum increases, and with them the number of individual damage sites caused by each one of the events. A dose effect curve for immediate DNA damage in tissue actually conforms to a linear “Impact-Number-Effectiveness Function” without threshold (Bond et al. 1995). This linear function is not identical in various cell types, and is lost as complex biological systems respond to low doses in various ways, as discussed below.
Within minutes after irradiation there is a plethora of DNA and chromatin modifications involved in DNA repair (Hall and Giaccia 2005). Immuno-histochemical methods now allow, for instance, the microscopic observation of DNA DSBs in individual cells (Rothkamm and Löbrich 2003; Sedelnikova et al. 2004; Neumaier et al. 2012). Well within 24 h, the fluorescent foci, supposedly indicative of DSBs, decrease to a lower number, closer to that of the background “spontaneous”, i.e. pre-irradiation, number (Rothkamm and Löbrich 2003). By way of these techniques one has learned that experimentally nonirradiated cells, depending on type and age, contain on average from about 0.1 to numerous DSBs at steady state, a finding that was strongly disputed for years (Sedelnikova et al. 2004). This value corresponds well to the calculated probability of 0.1 for a DSB to occur per average cell in the human body per day from endogenous, nonradiogenic sources (Pollycove and Feinendegen 2003). In contrast, at background radiation level, the probability of a radiogenic DSB to occur per day was calculated to be on average only about 1 in 10,000 cells. In other words, the calculated quotient of nonradiogenic to radiogenic DSBs produced per day in the human cell average amounts to about 1,000.
The capacity of normal cells to repair damage to DNA and other cellular components is genetically determined and may vary individually. Today, more than 150 genes have been described to be involved in DNA repair at high and low doses (Franco et al. 2005; Feinendegen et al. 2007a, 2008). Some genes are active only in low-dose stress responses; others again are modulated only after high doses (Franco et al. 2005; Mullenders et al. 2009; Tubiana et al. 2009). This reproducible data alone already contradicts the justification of the LNT-hypothesis for assessing health detriment as function of low dose. Moreover, low-dose irradiated confluent cells in culture appear to stall DNA repair until cell proliferation begins again (Rothkamm and Löbrich 2003). Indeed, an immediate induction of DNA repair in proliferating culture cells is reported to be elevated at low doses of about 1 mGy of X- and gamma radiation (Day et al. 2006; Mullenders et al. 2009; Tubiana et al. 2009).
In general, then, immediate damages of DNA provoke ready attempts at structural and functional reconstitution at the cell level. Radiation-induced effects in tissues are determined eventually by the degree of remaining DNA- and cell damage.
5 Hierarchy Level Responses in Biological Systems
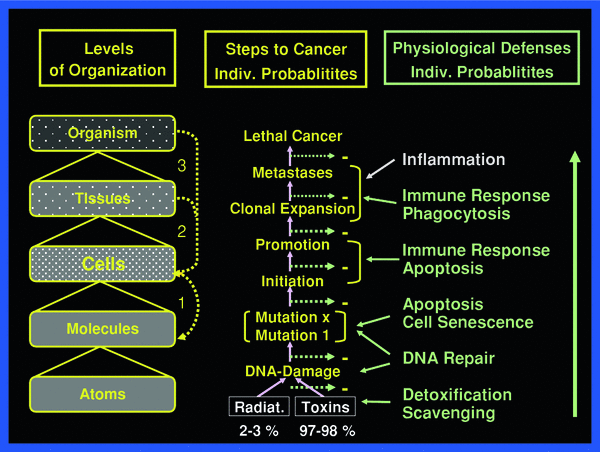
To fully appreciate the sequence of events to be reckoned with after irradiation, the body may be viewed as a composite of hierarchy levels of “protection” organization, as shown in Fig. 2.
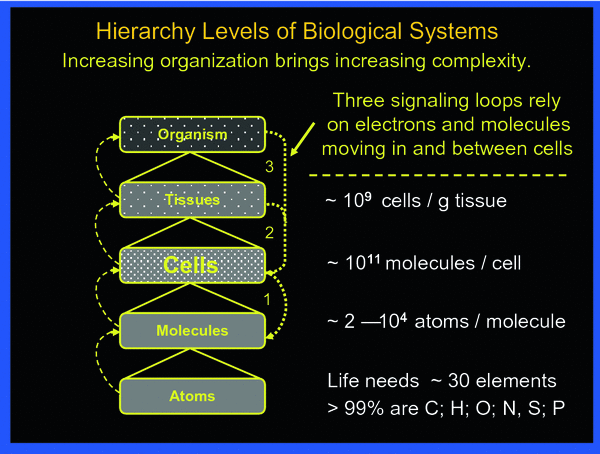

Fig. 2
The body may be viewed being organized in hierarchical levels with increasing complexity from bottom up. Intricate signaling within and between the various levels always involves cells. The three principal signaling loops assure functional integrity of the body in the face of abundant threats by toxic impacts from external and internal sources
Responses to the primary molecular perturbations and damages first involve the cells that have experienced one or more microdose events within a given period of time. The initially responding cells may transfer their perturbation or damage to neighboring nonirradiated cells causing so-called by-stander effects, which may be damaging and/or induce defenses (Mothersill and Seymour 2006). Similarly, energy deposition events in the intercellular matrix may affect nonirradiated cells (Barcellos-Hoff and Brooks 2001). These two damage categories are commonly referred to as nontargeted effects, in contrast, to targeted effects referring to the immediate damage in irradiated cells. If damage becomes lethal in many cells in a tissue, acute radiation effects may result in acute illness, the symptoms of which depend on the organ where cell death occurs. The degree of organ damage per unit dose largely depends on the response of the most sensitive organ–specific stem cells (Bond et al. 1966; Hall and Giaccia 2005; Fliedner et al. 2005). On the other hand, individual cells having escaped lethal radiation effects may still suffer malignant transformation and eventually cause cancer with metastases. The mechanisms of malignant transformation may include genomic instability induced in exposed cells and “handed down” to the cell’s progeny over several cell generations (Kadhim et al. 2006; Dziegielewski et al. 2008; Morgan and Sowa 2009).
Whereas the incidence of immediate DNA damage rises linearly with dose, damages to DNA and cells from both by-stander and matrix effects, and from genomic instability apparently have different dose thresholds, probably below 150 mGy, and, at least for by-stander effects reach plateaus with increasing dose at about 300–500 mGy. Immediate plus secondary damages to DNA and cells, i.e., targeted and nontargeted radiation damages, all induce the body’s defenses against such damaging events and against damage propagation to subsequent higher levels at tissues and the whole organism. The defense and protection systems also against nontargeted damage appear to add to the relatively low risk of radiogenic cell transformation, since epidemiology does not reveal cancer increase at doses below about 100 mGy (mSv).
6 Three Categories of Physiological Defenses of Complex Biological Systems
The extent of the targeted and nontargeted damage and its propagation in cells, tissues, and finally the whole-body depend on the type and degree of initial homeostatic perturbations and on the tolerance of homeostatic controls and defenses that operate at sequentially higher levels. Signaling loops coordinate controls within and between cells, between cells of different tissues and/or organs, and within the whole body, and all are subject to gene modulations (Guyton and Hall 2005). Therefore, certain defects in the involved genes may change individual susceptibility to radiation drastically.
One may, in general, discern three prototypes of defense: physical static ones, and two metabolic-dynamic ones, usually involving enzymes according to the individual’s genome.
The physical static barriers prevent impacts from changing matter, from disrupting a material structure and consequently its function in a system. For instance, a certain impact size, i.e., force, is required to move a body such as a stone on a surface in a given direction—well known and described by physical laws. Similarly, a certain target specific impact is needed to injure the skin, or to kill a cell, or to break an inter-atomic bond in a molecule. Moreover, tissue damage only occurs if a minimum number of cells that are essential to structure and function have been removed from their structural and functional places in tissue. Obviously, there are thresholds for a force to overcome a physical static barrier before an effect can be registered at the impacted object. With increasing magnitude of the impact, the effect becomes larger or more severe and eventually reaches a maximum. The corresponding Impact-Size-Effectiveness Function that describes the relationship between impact size and severity of effect gives graphically a sigmoid shaped curve (Bond et al. 1995).
Metabolic defenses can operate practically instantly at all levels of organization in normal organisms against potentially life-threatening events, which are shown schematically in Fig. 3. An example of defense at the tissue level presents the protection by the skin against manifold different types of impacts. If injured, the normal skin promptly initiates protective responses leading, for instance, to wound healing through signal-induced cell death, cell necrosis, phagocytosis, cell proliferation, and differentiation. At the molecular level, DNA damages of various kinds, be the base alterations, strand breaks or intermolecular linkages, induce a large number of specific repair responses (Hall and Giaccia 2005).
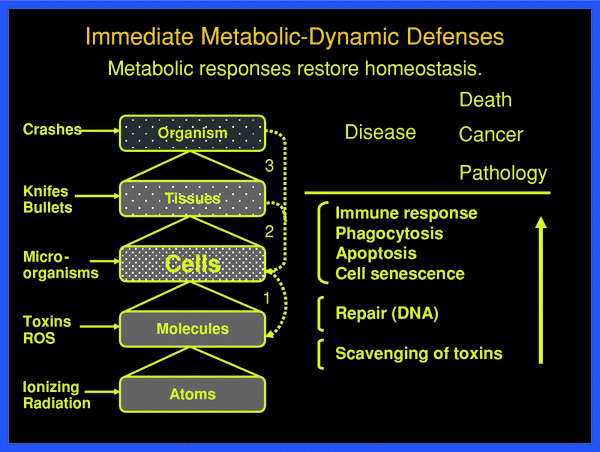

Fig. 3
Threats at the various organizational levels of the body are met by physical static and metabolic-dynamic defenses against damaging impact, damage creation and damage propagation. These defenses are successful if they restore homeostasis, from the molecular to the tissue-organ level. Only when the defense barriers are overcome, pathology develops with acute and late health effects, such as cancer. The individual defenses respond with individual probabilities
With a holistic view of systemic function at various levels one may distinguish the following prompt metabolic-dynamic defenses, as shown in Fig. 3. These may be put into three operational groups (Feinendegen et al. 1995, 1999, 2004, 2007a, b; Feinendegen and Neumann 2005):
a.
defenses by scavenging mechanisms at the atomic-molecular level;
b.
molecular repair, especially of DNA, with reconstitution of essential cell constituents, and functions;
c.
removal of damaged cells by induced cell death, i.e., apoptosis, cell necrosis, and an immediate immune response in an immunized body, with phagocytosis of killed cells, or by cell proliferation toward senescence.
In this context it is important to adhere to careful definitions. Thus, repair of a skin wound involves removal of damaged cells and cell debris as well as cell proliferation and differentiation. Therefore, terms like defense, repair, and damage removal must be linked to the levels where the damage occurs. Repair, damage removal, and replacement of damaged and/or lost molecules and cells in the course of tissue reconstruction for maintenance of tissue function usually are subsequently intertwined events.
Like the physical static barriers metabolic-dynamic barriers do not operate at a level always proportional to the degree of perturbation. In fact, these mechanisms of protection appear to allow perturbation to a certain degree before they begin to act to restore homeostasis, and thus prevent propagation of damage to successively higher levels of organization. This means, an impact must be large enough to overcome a threshold depending on the organizational level before structure and/or function are perturbed sufficiently to threaten the next higher level. There are many common daily examples with this principle response pattern.
In general then, only when homeostatic perturbations overwhelm structural and functional barriers at successive levels, from chemicals to molecules, to cells, to tissue, etc., disease can develop.
The above summarized cascade of defenses also operates against local damage and damage propagation from ionizing radiation. Because, increasing doses of ionizing radiation with large numbers of microdose events in the exposed tissues eventually overwhelm barriers at all hierarchical levels, high doses in large target volumes may allow damage at basic levels to propagate with minimal or no inhibition, and thus to evolve into clinically evident disease. As a consequence, many, but definitely not all, dose–response functions expectedly tend to be linear at higher doses, but not so at low doses.
There is a second metabolic-dynamic type of barrier which becomes activated by low-degree perturbations at a given level of biological organization. This barrier type is commonly referred to as stress response. It expresses an adaptation of the exposed system to better withstand renewed exposure to a potentially damaging impact by an agent that may be identical to the initial agent or mimics this agent. A common experience of this type of adaptation is the development of callus in a chronically burdened skin, or immunization for protecting the body against exposure to an infecting agent. Another example is properly conducted physical training to strengthen muscles and the cardiovascular system to improve physical endurance and/or athletic performance. A fourth example is properly dosed exposure to sunlight to induce tanning which will protect against a higher-degree exposure to sunlight by reducing the probability of sun burn, yet also may enhance the probability of skin tumors. Adaptive protections result from up-regulation of existing cascades of metabolic-dynamic barriers described above. In contrast to the promptly acting barriers; however, adaptive protections appear after a delay and increase to a maximum after one or repetitive stimulating impacts followed by a decline after the stimulus has disappeared. This decline is comparatively slow and may be observed for months to more than 1 year; some immunizations even protect for a lifetime.
Thus, low-dose irradiations, in contrast to high doses, can cause adaptive protections to function in cells, tissues, animals, and humans. There is a widespread misunderstanding of these low-dose induced adaptive protections only to act against renewed radiation and not to radiomimetic perturbations. Yet, radiation-induced adaptive protections operate also against the effects of other agents that may cause, for instance, DNA damage (Wolff et al. 1988), as referred to below.
7 Low-Dose Induced Adaptive Protections
Over the past three decades, experimental data in cultured cells and in animals have established that cells may respond very sensitively to low doses of low-LET type radiation by altering cell signaling (Zamboglou et al. 1981). This may lead with a delay of several hours after a single irradiation to the up-regulation of physiological defenses in terms of adaptive protection, discussed above (Feinendegen et al. 1999, 2004, 2007a, b; Mullenders et al. 2009; Tubiana et al. 2009). Up-regulation was quantified, for instance, regarding: scavenging of ROS that lasted for more than 10 h (Zamboglou et al. 1981; Feinendegen et al. 1984, 1995; Hohn-el-Karim et al. 1990); DNA repair lasting for several days (Olivieri et al. 1984; Wolff et al.1988); apoptosis to reach a maximum about 4 h after single exposure and to continue being elevated for more than 2 weeks following cessation of repetitive low-dose exposures (Kondo 1988; Fujita et al. 1998) and an increased immune response lasted for months and even more than 1 year with concomitant reduction, for instance, of metastases (James and Makinodan 1990; Tubiana et al. 2005, 2009). An integrated effect of adaptive protections shows in the degree of reduction of neoplastic transformations in cultured cells as well as primary cancer and metastases in animals following a single low-dose irradiation (Azzam et al. 1996; Feinendegen et al. 2004; Mitchel et. al. 2003, 2008; Elmore et al. 2009). In cultured cells a single low-dose, low-LET irradiation reduced neoplastic transformation to about 30 % of the transformation incidence in nonirradiated controls; and, a threshold for neoplastic transformation existed in such cells even after high cell doses from accelerated particles (Azzam et al. 1996; Elmore et al. 2009).
Like in the case of immediately protecting responses, adaptive protections do not necessarily develop proportionally to the degree of the perturbing event. Adaptive protections are related to dose in that they appear after single exposure at a low threshold of cell dose, increase to a dose around 100 mGy, then disappear as doses increase beyond 200 mGy of low-LET radiation and are hardly, or not at all, seen above about 500 mGy (Feinendegen et al. 1996, 1999, 2007a). An exception is apoptosis, in that its incidence apparently increases linearly over a certain dose region beyond single doses of 500 mGy, whereas at doses below about 100 mGy, there is evidence of apoptosis incidence to fall below the control level (Liu et al. 1996). In addition, unrepairable DNA damage obviously predisposes cells to induction of apoptosis more frequently than normal cells (Chandra et al. 2000). High-dose irradiation of mammals with alpha particles in vivo suggests induction of the body’s immune responses via the activation of immune cells in the neighborhood of high-LET damaged single cells (Harder 2008).
Adaptive responses are well known, for instance, following so-called oxygen stress (Chandra et al. 2000; Finkel and Holbrook 2000; Sen et al. 2000). They may be similar to those that in part are associated with radiation-induced adaptive protection (Hohn-el-Karim et al. 1990; Feinendegen et al. 1995, 2000; Feinendegen 2002). As referred to above, a normal average mammalian cell experiences a mitochondrial leak of about 109 ROS molecules per day, i.e. about 100 ROS molecules per millisecond, in the cytoplasm outside mitochondria, mainly from metabolic reactions; and additional small ROS bursts come from various responses to external cell signaling (Sen et al. 2000; Pollycove and Feinendegen 2003). An average microdose event, for instance produced by 100 kVp X-rays, creates about 150 ROS in the hit cell within a fraction of a millisecond. Both metabolic and radiation-induced ROS can trigger oxidative stress responses in terms of adaptive protections depending on concentrations, species, tissues, and cells (Finkel and Holbrook 2000; Feinendegen and Neumann 2000; Feinendegen 2002). In this context, normal background irradiation with its causing single microdose events per cell several times a year, as explained above, should be seen also as adjuvant for maintaining homeostasis (Feinendegen 2002), for instance by inducing apoptosis of predamaged cells (Chandra et al. 2000).
To repeat, adaptive protections were assumed initially to be confined to DNA repair following renewed irradiation (Olivieri et al. 1984; Wolff et al. 1988). Yet, it has become clear that the delayed stimulated protections may not only involve all physiological defenses but also operate against nonradiogenic damage, such as damage from endogenous toxins, like ROS (Chandra et al. 2000; Feinendegen et al. 1995) and from chemical mutagens (Wolff et al. 1988). Cells rarely can afford the energy “costs” associated with creating a special response to a rare or unique perturbation. The broad effectiveness of adaptive protections at all levels of biological organization, against both radiogenic and nonradiogenic damage, expresses a hormetic response, and is crucial in estimating probabilities of late radiation effects such as cancer, as will be discussed in more detail below.
The effect of cascades of homeostatic responses against propagation of primary damage at the DNA level to successive higher levels of the cell’s organization and tissues may be expressed by a set of equations shown schematically in Fig. 4.
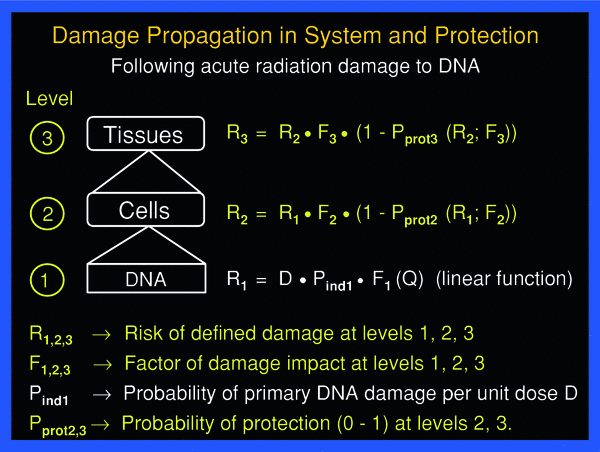

Fig. 4
Damage propagation to successive higher level of organization, from DNA, to cells, to tissue, and the effect of protection at the cell and tissue levels may be expressed schematically by a simple set of equations
8 Physiological Defenses Against Cancer
The various physiological barriers against damage and damage propagation sketched out above also operate in the course of oncogenesis, as illustrated in Fig. 5. Even if the protective mechanisms against cancer still are not fully understood, their effects are obvious. An illustrative example is the very low probability of a radiation-induced average DNA double-strand break in a potentially oncogenic blood-forming human tissue stem cell to bring about a lethal leukemia. This probability has been estimated to be close to 10−12 (Feinendegen et al. 1995). The claim that even a single DNA double-strand break, however grave, in a human stem cell may lead to cancer is scientifically unjustified.