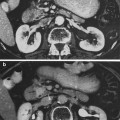
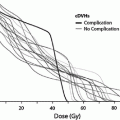
Fig. 1
Biocontinuum of adverse and late effects of the breast (with permissions from Rubin and Casarett 1968)
2 Anatomy and Histology
2.1 Gross Anatomy
2.1.1 Breast
The mammary gland consists of 15 to 20 lobes of glandular tissue with varying amounts of fat, in a dense fibroareolar stroma, and is attached to the anterior chest wall by pectoral fascia. In cross-sections starting from the nipple, there are openings of the lactiferous ducts and their lactiferous sinuses, which are the conduits for the secretions of the hormonally stimulated breast glands. These ducts are distinct and individual for each lobule; they first run dorsally from the nipple and then spread radially into the glandular tissue (Fig. 2a).
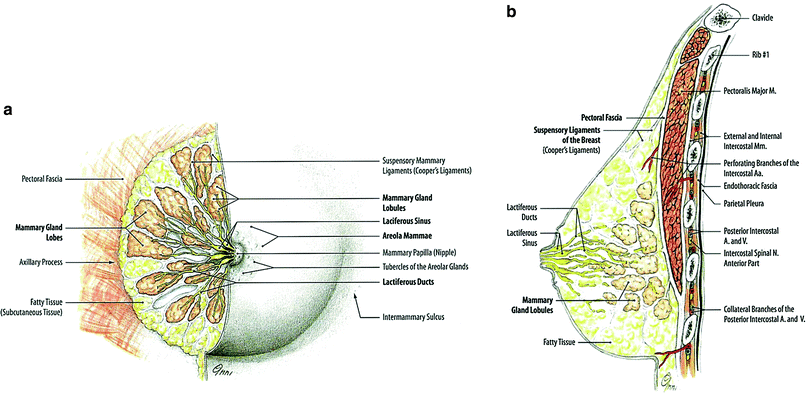

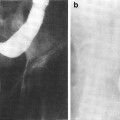
Fig. 2
a Right mammary gland of a woman before menopause. Glandular and fatty tissue and the efferent duct system have been dissented into the lateral half. b Sagittal section through the mammary gland and the anterior thoracic wall near a woman’s nipple (reprinted with permission from Tillman 2007)
2.1.2 Breast Relative to Surrounding Connective Tissue
The female breast extends in the superior-to-inferior direction from approximately the 2nd through 6th ribs. Posterior (or “deep”) to the breast is the pectoral fascia overlying the pectoralis major muscle. The lateral aspect of the pectoralis major makes up most of the anterior wall of the axilla.
Deep to the pectoralis major muscle is the pectoralis minor muscle. The pectoralis minor is shaped like a triangle, is nearly completely covered by the pectoralis major (Fig. 2b). The borders of the pectoralis minor muscle are used to (somewhat arbitrarily) define the anatomic levels of the axilla (level I is lateral, level II is deep, and level III is medial, to the muscle, respectively).
The serratus anterior muscle is a broad sheet that overlies the lateral part of the chest wall and forms the medial wall of the axilla.
The Pectoralis major and minor muscles, serratus anterior, along with the small subclavius muscle that is inferior to the clavicle, comprise the anterior axioappendicular muscles that are important in shoulder and upper humerus movement. Table 1 summarizes each of these movements. The intercostal muscles span from rib to rib. The primary role for the intercostals muscles is in respiration to support or provide rigidity for the intercostals spaces, resisting negative and positive intrathoracic pressures. (The diaphragm is the primary muscle of respiration.)
Table 1
Chest wall musculature that effects shoulder motion: anatomy and function
Chest wall muscles | Attachments | Innervation | Main Action | |
Proximal | Distal | |||
Pectoralis major | Clavicular head: Medial clavicle | Upper humerus | Pectoral nv. C5, C6 | Adducts and medially rotates humerus Draws scapula anterior and inferior Clavicular h.: flexes humerus Sternocostal h.: extends humerus from flexed position |
Sternocostal head: Manubrium, sternum Upper six costal cartilages | Upper humerus | C7, C8, T1 | ||
Pectoralis minor | 3rd–5th ribs medially | Coracoid Scapula | Medial pectoral nv. C8, T1 | Stabilizes scapula Draws scapula inferior and anterior |
Subclavius | 1st rib-costal junction | Mid-clavicle | Nv to subclavius C5, C6 | Anchors, depresses clavicle |
Serratus anterior | 1st–8th rib, anterior-laterally | Scapula, medial border | Long thoracic nv. C5, C6, C7 | Depresses scapula Holds scapula against thoracic wall Rotates scapula |
Intercostal | Rib—superior border | Rib—inferior border | Intercostal nv. | Stabilize ribs during expiration |
2.2 Histology
2.2.1 Breast
These changes are well described by Zhang (1999) as follows: “The terminal portion of the tubuloalveolar glands shows several lobules with numerous branching ducts lined by epithelial cells. The epithelial cells of the ducts appear to be more often transformed into cancer (i.e. ductal carcinoma) than are the corresponding cells of the lobules (i.e. lobular carcinoma). With pregnancy and lactation, there are marked hormone-mediated cellular changes. Declining ovarian function at menopause leads to cellular regression” (Fig. 3a, b).
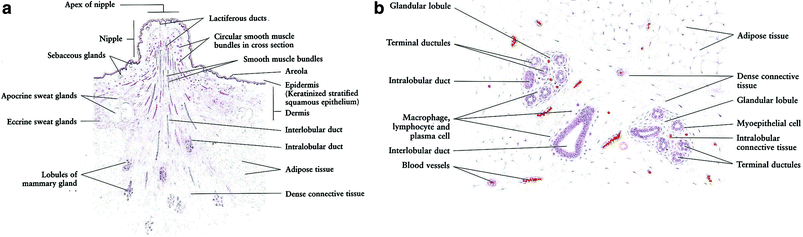

Fig. 3
a This figure shows a resting mammary gland, in which the glandular tissues are not developed. The lobules are composed of only ducts, dense connective tissue, and adipose tissue. (reprinted with permission from Zhang 1999) b In the resting mammary gland, the glandular tissue is not active, as it would be during pregnancy or lactation (reprinted with permission from Zhang 1999)
2.2.2 Chest Wall Muscle
The skeletal muscles of the chest wall connect to the humerus, scapula, clavicle, and ribs via tendons, and contract and relax to coordinate shoulder movement. Muscles are composed of bundles of single large cells (called muscle fibers) that form by cell fusion and contain multiple nuclei. Each muscle fiber contains many myofibrils, which are bundles of actin and myosin filaments organized into a chain of repeating units called sarcomeres. Individual muscle fibers are surrounded by endomysium, that carries numerous capillaries to supply blood to the muscle fibers. See “Musculoskeletal System: Growing Endochondral Bone, Mature Osseous, Muscle (Striated), and Soft Tissue Mesenchyme” Musculoskeletal.
3 Physiology and Pathology
3.1 Physiology
These changes are well described by Zhang (1999) as follows: “During pregnancy, the mammary glands undergo extensive histological changes in preparation for lactation. These changes are primarily manifested in two aspects: the formation of secretory acini and the relative decrease of connective tissue and adipose tissue”.
“Under the influence of several hormones, such as estrogens, progesterone, prolactic, and human placental lactogen, the epithelium of the terminal ductules proliferates to form numerous secretory glandular acini. These are spherical in shape and composed of a layer of cuboidal or columnar epithelial cells resting on a basement membrane. Stellate myoepithelial cells are found between the acinar epithelial cells and the basement membrane.”
“At the end of pregnancy and after parturition, the glandular tissues reach their highest development and begin to secrete milk. Figure 4a shows a lactating mammary gland with its glandular tissue at the state of maximum development. The glandular acini are lined by a cuboidal epithelium, and some epithelial cells have secretory vacuoles within their cytoplasm. The myoepithelial cells are located between epithelial cells and basement membrane, and their contraction pushes milk toward the excretory ducts. The acinar lumina are dilated and filled with secretion of milk.”
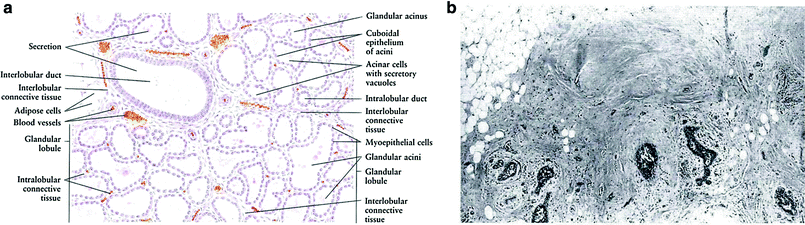

Fig. 4
a This figure shows a lactating mammary gland with its glandular tissue at the state of maximum development (reprinted with permission from Zhang 1999). b This photomicrograph of an irradiated female breast shows severe diffuse fibrosis of the breast stroma, especially encircling lobules and small ducts. The epithelium of each lobule and ductule is quite distorted by the fibrosis. This epithelium is atrophoid. but significant atypism is not observed. Much of the lobular epithelium is lost entirely. The fibrosis shows homogeneous hyalin change characteristic but not diagnostic of radiation-induced fibrosis. Several enlarged, hyperchromatic cells in the scar may be atypical fibroblasts but more likely are remaining altered lobular gland epithelium. Low power, H & E (with permissions from Fajardo 2001)
3.2 Biology
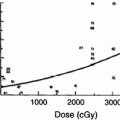
The pathogenesis of radiation-induced muscle injury has been studied only to a limited extent in animal models. In rat thighs, there was an increase in release of amino acids 4–6 h after a single dose of 15 Gy, suggesting acute protein breakdown (Schwenen et al. 1989). In rabbits, 24 h after a single dose of 11–13 Gy to the pectoralis muscle interfibrillar edema, myofilament disruption, and endothelial swelling were present on electron microscopy. At 1 week and 1 month, there were areas of myofiber necrosis, focal atrophy, fiber hypertrophy, mesenchymal cell proliferation, and fibrinoid necrosis of the vessels. Significant vascular changes were present and confined to the microvasculature (Khan 1974). Similar findings of muscle fibrosis, vessel lesions, and inflammation were observed in muscles of pigs steadily worsening over 6 months after 40–80 Gy given in a single dose. Increased collagen and glycosaminoglicans contents were found in both fibrotic and perifibrotic muscle tissue. Cultures of fibroblasts from fibrotic tissue demonstrate that these continue to be metabolically more active for several months post-irradiation than fibroblasts from nonirradiated tissue (Remy et al. 1989; Wegrowski et al. 1988). Also, a two- to four-fold increase in transforming growth factor Beta (TGF-β) was found in a pig model within fibrotic tissue as early as 3 weeks after 35 Gy and was still increased 1 year later. The TGF-β was localized to myofibroblasts, mononuclear inflammatory cells, and endothelial cells as well as cells within the collagenous matrix (Martin 1993).
Adult skeletal muscle is a stable tissue with little turnover of nuclei. However, in response to injury, skeletal muscle has the ability to complete extensive and rapid regeneration (Charge and Rudnicki 2004). Repair occurs through differentiation of myogenic precursor cells or satellite cells. Satellite cells are present in all skeletal muscles in varying amounts. The initial phase of muscle repair is characterized by necrosis of the damaged tissue, followed by an inflammatory response, and subsequent activation of a muscle repair process. Muscle regeneration is regulated by numerous secreted factors including TGF β, which has inhibitory role. A recent study demonstrated that doses of gamma radiation >/ = 5 Gy reduced satellite cell numbers by at least 70 % due in part to elevated apoptosis and the inhibition of cell cycle progression (Caiozzo et al. 2010 ).
These studies suggest that radiation causes injury to myofibers and muscle microvasculature, decreases satellite cells, increases TGF-β and other inhibitory mediators, leading to less regenerative capacity and increased fibrosis. The replacement of myofibers with fibrosis leads to a shorter and weaker muscle and impacts patient function.
3.3 Pathology
The major overt-radiation induced changes are those to skin, ranging from erythema to dry, then moist desquamation. The late changes are pigmentation, fibrosis, and telangiectasia. These physiopathologic changes are well documented and illustrated in the integument/skin chapter.
The alterations to the breast tissue described in biopsies and mastectomies, months to years post-radiation, with or without chemotherapy, include the following findings (example see Fig. 4b).
The most consistent change is in the terminal duct-lobular unit, where atypical epithelial cells with large hyperchromatic nuclei and small nuclei are seen.
Fat atrophy due to loss of adipocytes after standard radiation doses accounts for the initial improved cosmetic lift. However, with larger doses, post-irradiation lumps may occur which are due to fat necrosis. The histologic appearance is foam cells, foamy multinucleated giant cells and zones of fibrosis. Retraction and deformation can occur in areas of surgical lumpectomy sites when large fraction boost doses are administered (Clarke et al. 1983).
Fibrosis varied with perilobular and intralobular distribution. Stromal fibrosis, atypical fibroblasts, and vascular lesions occur. The severe diffuse fibrosis of breast stroma, tends to encircle lobules and small ducts. The epithelium of each lobule and ductile is distorted by the fibrosis.
Lactation can occur; however, the breast does not enlarge during pregnancy.
Ductal cell atypia rimmed with bipolar nuclei of myoepithelium or epithelial cell nuclei may be large. The need to differentiate recurrent cancer from cell atypia is a challenge but monoacinar arrangements, anisocytosis, and anisonucleosis can usually determine benign diagnosis as correct. Small needle biopsy aspirations can be a challenge but true recurrence usually resembles the original carcinoma.
The pathophysiology of radiation injury to skeletal muscle is characterized by early muscle injury and inflammation and late fibrosis mediated predominantly by vascular injury (See Chap. 35 Musculoskeletal).
4 Clinical Syndromes (Endpoints)
Acute effects from fractionated radiation treatment to the breast involving the skin include erythema and moist dermatitis, and are illustrated in Fig. 5 in the skin/integument chapter. The late changes involving skin are relatively infrequent but can include fibrotic scarring and retraction of the breast, illustrated in Figs. 6 and 7 in the skin/integument chapter. A variety of endpoints can be used to grade the effects of therapy on the breast (Table 2).
Table 2
Breast toxicity assessment: LENT SOMA
Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|
Subjective | ||||
Pain | Occasional and minimal Hypersensation, Pruritus | Intermittent and tolerable | Persistent and intense | Refractory and excruciating |
Objective | ||||
Edema | Asymptomatic | Symptomatic | Secondary dysfunction | |
Fibrosisa/Fat necrosis | Barely palpable increased density | Definite increased density and firmness | Very marked density, retraction, and fixation | |
Telangiectasia | <1 cm2 | 1–4 cm2 | >4 cm2 | |
Lymphedema arm (circumference) | 2–4 cm increase | >4–6 cm increase | >6 cm increase | Useless arm, angiosarcoma |
Retraction/Atrophyb | 10–25 % | >25–40 % | >40–75 % | Whole breast |
Ulcer | Epidermal only, ≤1 cm2 | Dermal, >1 cm2 | Subcutaneous | Bone exposed, necrosis |
Management | ||||
Pain | Occasional non-narcotic | Regular non-narcotic | Regular narcotic | Surgical intervention |
Edema | Medical intervention | Surgical intervention/mastectomy | ||
Lymphedema arm | Elevate arm, elastic stocking | Compression wrapping, intensive physiotherapy | Surgical intervention/amputation | |
Atrophy | Surgical intervention/mastectomy | |||
Ulcer | Medical intervention | Surgical intervention, wound debridement | Surgical intervention/mastectomy | |
Analytic | ||||
Photographs | Assessment of skin changes as atrophy, retraction or fibrosis, ulcer | |||
Tape measure | Assessment of breast size and forearm diameter | |||
Mammogram | Assessment of skin thickness and breast density | |||
CT/MRI | Assessment of breast size, fat atrophy, fibrosis density | |||
4.1 Breast
4.1.1 Cosmesis (Late Breast Appearance)
The main goals of breast conservation therapy in early-stage breast cancer are to provide primary tumor control comparable to mastectomy and to preserve an acceptable cosmetic appearance of the breast. An unsatisfactory cosmetic outcome should be considered as a potential late toxicity. The rate of poor or fair cosmetic outcome in most series is 15–20 % or less (Pezner et al. 1992; Ryoo et al. 1989; De la Rochefordiere et al. 1992; Taylor et al. 1995; Wazer et al. 1992; Harris et al. 1979; Olivotto et al. 1989; Sneeuw et al. 1992). It has been demonstrated in many studies that surgical factors including the extent or volume of surgical resection (Taylor et al. 1995; Wazer et al. 1992) and scar orientation, (Taylor et al. 1995) have a large impact on cosmetic outcome (Pezner et al. 1992; Ryoo et al. 1989; De la Rochefordiere et al. 1992; Taylor et al. 1995; Wazer et al. 1992; Tuamokumo and Haffty 2003). The use of chemotherapy and patient factors such as breast size, older age, and race have also been associated with more frequent cosmetic failures (Pezner et al. 1992; Ryoo et al. 1989; De la Rochefordiere et al. 1992; Taylor et al. 1995; Wazer et al. 1992; Tuamokumo and Haffty 2003). However, several radiation treatment factors are associated with poorer cosmetic outcomes as well. It is important to consider these factors when planning radiation treatment to minimize the late toxicity rate.
Table 3 lists the cosmetic outcomes from single institution retrospective studies that have analyzed the impact of radiation techniques on subsequent cosmetic outcome. Wazer et al. from New England Medical center at Tufts demonstrated an increase in fair/poor cosmetic outcomes with larger chest wall separations (24 cm mean) and greater maximal dose inhomogeneity (13 % mean) at the central axis (Wazer et al. 1992). The use of a boost and a supraclavicular and/or axillary field were the other factors associated with a higher proportion of fair/poor cosmetic outcomes. In this study, an electron boost, but not an interstitial implant boost, was associated with the decline in cosmetic outcome. Patients in this study were treated with 6 MV photons, 81 patients with an implant boost, and it is not stated what proportion were treated to >2 fields.
Table 3
Physician assessed cosmetic results from breast conservation therapy (White and Joiner 2006 with permission)
Institution | N | F/U (years) | Excellent (%) | Good (%) | Fair (%) | Poor (%) | Radiation factors associated with poorer cosmesis |
|---|---|---|---|---|---|---|---|
Tufts U46 | 234 | 4.2 | 41 | 47 | 9 | 3 | Heterogeneous RT dose; boost; use of >2 fields |
Harvard/JCRT44, 49 | Breast dose >50 Gy; use of >2 fields; boost dose >18 Gy; implant boost | ||||||
<I981 | 504 | 8.9 | 58 | 28 | 10 | 4 | |
1982–1985 | 655 | 5.6 | 73 | 23 | 3.5 | 0.5 | |
Washington U45 | 458 | 4.4 | 38 | 44 | 15 | 4 | Use of >2 fields; breast dose >50 Gy; no compensator filters |
The effect of radiation technique on the cosmetic result was demonstrated by the Joint Center for Radiation Therapy and Harvard Department of Radiation Therapy when it compared cosmetic results in two different cohorts of patients treated between 1970–1981 and 1981–1985 (De la Rochefordiere et al. 1992). In the earlier cohort, 85 % received a 3-field technique, 95 % an implant for boost, 33 % ≥ 50 Gy breast dose, and 85 % > 18 Gy boost dose. The institution had previously found that the use of >2 fields, an implant boost, boost dose >18 Gy, and a breast dose >50 Gy were associated with poorer cosmetic outcomes (Harris et al. 1979; Olivotto et al. 1989). Treatment techniques had changed during the latter time interval such that 55 % received a 3-field technique, 47 % an implant for boost, 5 % ≥ 50 Gy breast dose, and 42 % > 18 Gy boost dose. The cosmetic results were better with the techniques used in the latter period (Table 3). When examined, there was no influence of the boost, number of fields treated, and/or the daily dose on cosmetic outcome in the latter cohort (De la Rochefordiere et al. 1992).
Washington University similarly found that the percentage of excellent/good cosmetic outcomes decreased with the use of more than 2 fields (P = 0.034), and increasing radiation dose to the entire breast (P = 0.024). With increasing separations at the central axis, a relative deterioration occurred in excellent/good rations, especially with the use of lower energy, 4 MV photons. This is inferred to be from the dose inhomogeneity that occurs with the larger chest wall separation. The effect of dose homogeneity on cosmetic outcome is again demonstrated in this study by a significantly higher frequency of excellent/good cosmetic scores (82 %) that occurred with the use of compensating filters compared with no use of compensating filters (59 %) (P = 0.002). Daily fraction size (1.8 vs. 2.0 Gy), boost versus no boost, and the type of boost did not influence cosmetic outcome in this series (Taylor et al. 1995). Other studies have confirmed the influence of radiation therapy factors on cosmetic outcomes. For instance, Ryoo et al. (1989) reported that the use of a wedge in the breast tangents was a significant factor for obtaining a good cosmetic result.
The cosmetic failure rates reported in all of these studies reflect treating physician observation. Studies that include patient-rated cosmetic evaluations demonstrate fairly good concordance with physician-rated cosmesis and satisfaction with a range of cosmetic outcomes (Taylor et al. 1995; Sneeuw et al. 1992).
In an attempt to objectively measure cosmetic outcome, Pezner et al. developed a breast retraction assessment (BRA) that quantified the amount of retraction of the treated breast in comparison to the untreated one by measuring the lateral and vertical displacement of the nipple (Pezner et al. 1992). On multivariate analysis, in order of descending importance, patient age >60, extensive breast reaction, patient weight >150 pounds, and upper quadrant primary site were the most significant factors related to breast retraction after breast conserving surgery and radiotherapy (BCT). None of the radiotherapy (RT) parameters studied were associated with breast retraction. Subset analysis related that the volume of the boost had some relation to retraction, but did not reach statistical significance.
Cosmetic outcome was assessed in a subset of 101 women accrued to the Milan III trial that randomized women with breast cancers ≤2 cm in size to quadrantectomy (QUAD) or quadrantectomy plus breast irradiation (QUART) (Ververs et al. 2001; Veronesi et al. 2001; Sacchini et al. 1995). Radiation consisted of 50 Gy whole breast dose followed by a “scar” boost of 10 Gy, all delivered with 2 Gy fractionation. Interestingly, there were no statistically significant differences in cosmetic outcome (scored either objectively based on nipple displacement/symmetry or subjectively by physicians and patients) between the QUAD versus QUART groups (Sacchini et al. 1995). The negative findings from this study are in conflict with many other studies. These differences might be due to the small sample size or the extent of the surgery. For example, the negative cosmetic effects of a QUAD (versus a lesser degree of surgery) might make the potential effects of radiation less evident.
The effect of the boost on the cosmetic result was evaluated in two randomized trials. In EORTC 22881/10882 (Vreilng et al. 1999), 5,569 patients with stage I and II breast cancer who had received lumpectomy, axillary dissection, and breast radiation up to 50 Gy over 25 fractions were randomized between a boost of 16 Gy or no boost if the lumpectomy resection margins were negative. Cosmetic outcome in each arm was assessed by two methods postoperatively and at 3 years: by a five-physical panel evaluating photographs in a sample of 713 patients (Table 4) and by the percentage BRA relative to a reference length in a sample of 1,141 patients. Postoperatively, there was no significant difference in cosmetic assessment between the two arms. However, by 3 years, the patients in the boost arm had significantly lower rate of excellent/good cosmetic outcome and nearly double the rate of fair outcomes (13 % no boost vs 25.8 % boost) (P = 0.0001). Very few patients in either arm had a poor result at either time period. By the panel assessment, the boost group had significantly worse median scores for all the items evaluated—appearance of the surgical scar, breast size, breast shape, nipple position, and areola shape. Interestingly, despite the observations of the panel, the difference in the percentage BRA was small, less than 1 % between the borderline statistical significance (P = 0.04). In the Lyon trial, patients with early breast carcinoma (≤3 cm) treated by local excision (tumorectomy or quadrantectomy), axillary dissection, and conventional 50-Gy irradiation were randomized to either a boost of 10 Gy electrons, or no boost. There were no significant differences in either the physician-evaluated or patient self-assessment of cosmetic outcome.
Table 4
The impact of tumor bed boost on cosmetic outcomes in randomized trials
EORTC 22881/10882 (n = 713) | Lyon trial Romestaing et al. (1997) | |||||||
|---|---|---|---|---|---|---|---|---|
Post-op | 3 year Follow-up | 2 year by physician (n = 702) | 2 year by patients (n = 600) | |||||
Score (%) | No boost | Boost (16 Gy) | No boost | Boost (16 Gy) | No boost | Boost (10 Gy) | No boost | Boost (10 Gy) |
Excellent | 37.4 | 34.8 | 41.7 | 32.7 | 85 | 90 | ||
Good | 47.3 | 45.0 | 43.9 | 38.2 | ||||
Fair | 13.7 | 18.8 | 13.1 | 25.8 | Not stated | Not stated | Not stated | Not stated |
Poor | 1.6 | 1.4 | 1.4 | 3.3 | 0 | 0 | ||
In a prospective randomized trial, the use of IMRT was associated with a better late cosmetic outcome (Table 5; Donovan et al. 2007). Almost all of these patients had RT to the breast alone, without the nodes. Similarly, IMRT has been demonstrated in a randomized trial to reduce acute effects as well; e.g. rate of moist desquamation reduced from ≈45 % without IMRT to ≈30 % with IMRT (Pignol et al. 2008).
Table 5
Proportion of patients showing change in breast appearance after randomization to 2D (control group) or IMRT (test group)a
Standard 2D | IMRT | |
|---|---|---|
1 year | ||
Percent with no effects | 64.1 | 74.2 |
Percent with mild effects | 28.2 | 21 |
Percent with marked effects | 7.6 | 4.8 |
2 year | ||
Percent with no effects | 56.6 | 65.1 |
Percent with mild effects | 38 | 30.2 |
Percent with marked effects | 5.4 | 4.7 |
5 year | ||
Percent with no effects | 41.8 | 60.2 |
Percent with mild effects | 44.3 | 29.7 |
Percent with marked effects | 13.9 | 10.2 |
In summary, several patient and treatment factors can contribute to a poor cosmetic outcome following breast conservation therapy with RT. The radiation factors that influence the cosmetic outcome in most series are the use of a boost, greater than 2 fields (i.e., the addition of a supraclavicular, axillary, or internal mammary field), the total dose, and dose heterogeneity in the breast fields. Newer radiation therapy planning methods such as 3-dimensional conformal therapy or intensity-modulated radiation therapy can produce more homogeneous dose distributions through the breast.
The negative cosmetic impact of the boost needs to be taken in the context of the increased rate of local control achieved when the boost is added; a meaningful benefit especially in some patient subsets. Nevertheless, care should be taken that the boost is delivered using techniques that minimize morbidity. Careful image-guided localization of the cavity will help reduce excess breast tissue being taken to higher doses unnecessarily.
4.1.2 Chronic Breast Pain
A survey of 127 breast cancer survivors, on average 3 years post-treatment, revealed that 27 % reported chronic pain (Carpenter et al. 1998); rated as mild in 90 % of patients. The sites of pain affected were breast 86 %, ipsilateral arm 69 %, and ipsilateral axilla 81 %. Pain in all three sites was reported in 58 %. The prevalence of pain was 27 % after lumpectomy with RT, and 23 % after mastectomy alone.
The impact of irradiation on breast pain has been reported form two randomized studies. In a prospective randomized trial at Princess Margaret Hospital of tamoxifen alone versus tamoxifen plus breast RT, in women aged ≥50 who have had a lumpectomy, the addition of radiation did not adversely affect breast pain up to 12 months post-treatment (Ryan et al. 2003).
In a large prospective study conducted in Ontario, Canada, women with lymph node negative breast carcinoma who had undergone lumpectomy and axillary lymph node dissection were randomized to either breast irradiation or no further treatment. Within the first few months or randomization, the irradiated patients had a lesser quality of life (QOL) and more breast pain, than did the control group, with the maximum difference at about 3–6 months post-randomization. The differences declined over time, and became undetectable by 2 years (Whelan et al. 2000). Similarly, there was no difference in long-term patient-reported QOL metrics in the randomized trial of ± IMRT (Donovan et al. 2007).
In concert, these data suggest that long-term breast pain can occur, but that it is not a prominent clinical problem for most patients.
4.1.3 Lactation
There are several studies that access the ability of the breast to lactate following breast conservation therapy with lumpectomy plus RT (Table 6). In general, normal lactation is not possible (e.g., reduced frequency or volume of lactation).
Table 6
Reports of lactation in irradiated breasts (following breast conservation with tumor excision and breast RT for early-stage breast cancer)
Author | Report type | Tx year | Patient age | T stage | ALND | Dosec (Gy) | Boostd (Gy) | RNI | Chemo | Interval between RT and pregnancy (year) | Breast enlargement during pregnancy | Colostrum present | Breast milk start day postpartum | Lactation duration (months) | Lactation rate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Findlay et al. (1988) | Case | 1979 | 35 | NS | Yes | 48.6 | 20 | No | No | 4 | NS | No | 3 | 2.5 | NA |
Rodger et al. (1989) | Case | 1983 | 30 | T1a | Yes | 42–45 | 20 | Yesg | NS | 3 | NS | NS | NS | NS | 1/2a |
Green (1989) | Case | 1988 | 32 | T2 | Yes | 48.6 | No | No | NS | 19 mo | No | No | 5 | 1 | NA |
Higgins and Haffty (1994) | Retrospective review | 1965–1989 | 25–41 | I–II | Yes | 50–54 | 10–11 | No | 1 pt | 28–75 mo | NS | NS | NS | 1–7 | 4/11a |
Tralins (1995) | ASTRO survey | 1995 | 30–39 | T1–2 | NS | 40–50.4 | 10–22 | NS | 3 pts | 3b | Noe | Yesf | 7h | NS | 18/53a |
A cohort study compared the breast feeding outcomes in 83 female survivors of Hodgkin’s lymphoma (treated with mediastinal RT to a median dose of 41 Gy, range 27–46 Gy) to their 70 female sibling controls. Both groups had 141 live births and 94 attempts at breastfeeding. In the irradiated group, 57/94 (61 %) of these attempted breast-feedings were successful versus 74/94 (79 %), p = 0.04. There were no meaningful differences noted in their analysis related to various factors including age at the time of RT, age at the time of pregnancy, nor RT dose. There were 30 patients ≪21 years of age at the time of their initial diagnosis. They had 49 live births, 35 attempts at breastfeeding, and of these 66 % (23/35) were successful. On a per-person (rather than per-birth basis), 22/30 patients attempted to breast feed, and 68 % (15/22) were successful at least once. Of these, there were eight girls aged 14–16 at diagnosis. All of them attempted breastfeeding, and 6/8 were successful (McCullough et al. 2010).
The apparent greater success at breast feeding in the Hodgkin’s survivors (compared to the breast cancer patients) may be related to the lack of breast surgery, the often-used lower doses of RT, and the fact that the entire breast is not usually irradiated.
4.2 Chest Wall and Associated Muscle
4.2.1 Shoulder Symptoms/Mobility
Patient-reported shoulder-associated complaints (e.g. pain and stiffness) are common clinical syndromes following breast cancer therapy. Table 7 summarized several studies comparing outcomes following therapy with mastectomy versus breast conservation. Globally, the outcomes with breast conservation (that almost always included RT) were similar or better than those with mastectomy.
Table 7
Rates of patient-reported shoulder symptoms following breast cancer treatment
Author (year) | N | Surgerya | RT | Symptom Reported | Rate of symptoms in patients undergoing mastectomy (%) | Rate of symptoms in patients undergoing breast conservation therapy (%) | P f |
|---|---|---|---|---|---|---|---|
Ernst et al. (2002) | 148 | MRMb 106 BCTc 42 | All (no RT axilla) | Paind >3 Strengthd <6 | 27 14 | 24 9 | ns |
Nesvold et al. (2008) | 263 | MRM 186 BCT 77 | All (23 % RT Axilla) | Paine 3–5 Shoulder stiffnesse 3–5 | 58 32 | 33 12 | 0.001 0.001 |
Kärki et al. (2005) | 96 | MRM 64 BCT 32 | 63.5 % | Neck-shoulder pain Shoulder movement restriction | 42.2 17 | 37.5 5 | – – |
Similarly, Table 8 summarizes some studies comparing outcomes based on objective measures of shoulder mobility, in patients undergoing mastectomy versus breast conservation therapy. In the breast conservation patients, breast RT was delivered in almost all cases, and axillary RT was delivered in only a minority of patients. Across the board, the outcomes appear superior with breast conservation versus mastectomy.
Table 8
Rates of measured reduction in shoulder range of motion (ROM) following breast cancer treatment
Author (year) | N | Surgerya | Rate of RT usage in BCT patients (axillary irradiation usage) | Measure | Rate of symptoms in patients undergoing mastectomy (%) | Rate of symptoms in patients undergoing breast conservation therapy (%) | P d |
|---|---|---|---|---|---|---|---|
Ernst et al. (2002) | 148 | MRMb 102 BCTc 46 | All (no RT axilla) | ROM >20° | 15 | 3 | ns |
Nesvold et al. (2008) | 263 | MRM 186 BCT 77 | All (23 % RT axilla) | ROM >25° Flexion Abduction | 24 38 | 7 18 | 0.01 0.01 |
Gosselink et al. (2003) | 76 | MRM 31 BCT 45 | 84 % (16 % RT axilla) | ROM >25° Flexion Abduction | 87 90 | 58 53 | 0.001 0.001 |
Voogd (2003) | 332 | MRM 118 BCT 213 | 64 % (no RT axilla) | Abduction >20° | 14 | 7 | 0.03 |
Sugden et al. (1998) | 141 | MRM 27 BCT 80 | All (35 % RT axilla) | Any ° ROM Pre-RT Post-RT | 44 79 | 9 35 | 0.001 0.001 |
The addition of RT clearly can have negative effects on shoulder mobility. Table 9 summarizes several studies comparing outcomes, both patient-reported and objective measures, in patients undergoing surgery with or without RT. The addition of RT is associated with a higher rate of impairment.
Table 9
Incidence of impaired shoulder mobility after irradiation (White and Joiner 2006 with permission)
Institution (author) | N | Surgery | Nodal RT (%) | Measure | Surgery (%) | Surgery + RT (%) |
|---|---|---|---|---|---|---|
Netherlands Cancer Institute (Bijker) | 691 | Mastectomy | 70 | Patient self-report | 3.4 | 8a |
18.9b | ||||||
University of Oxford (Sugedn) | 39 | Mastectomy | 35 | Measured ROMc | 71d | 81d |
102 | Lumpectomy | 31e | 59e | |||
Aarhus University Hospital (Hojris) | 84 | Mastectomy | 100 | Measured ROM | 2 | 16 |
Odense University (Ryttov) | 57 | Mastectomy | 23 | Measured ROM | 6.8 | 38 |
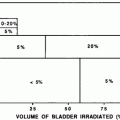
The extent of the RT field is also important, as can be inferred from the data summarized in Table 10. The studies listed compare outcomes, both patient-reported and objective measures, in patients undergoing surgery with or without RT, with variable extents of the RT field. The rates of impairment appear typically higher in the patients receiving RT to the breast and axilla versus those undergoing RT to the breast alone (where the impairment rates are similar to those patients undergoing surgery without RT).
Table 10
Rates of reduction in shoulder range of mobility (ROM): with or without radiotherapy (RT)
Author (year) | N | Surgerya | Measure | Rate in patients without RT | Rate in patients with RT (extent of RT as noted) | P b |
|---|---|---|---|---|---|---|
Johansson et al. (2001) | 61 | 26 MRM 35 BCS | Reduced ROM >10° | 23 % | 79 % with RT to the axilla 19 % with RT to the breast alone | <0.001 ns |
Hojris (2000) | 84 | 84 MRM | ROM <175° | 15 % | 52 % (with nodal RT) | <0.01 |
Sugden et al. (1998) | 107 | 39 MRM 102 BCS | Any reduction in ROM |