Definitions of TNM
Primary tumor (T)a
TX
Primary tumor cannot be assessed
T0
No evidence of primary tumor
Tis
High-grade dysplasiab
T1
Tumor invades lamina propria, muscularis mucosae, or submucosa
T1a
Tumor invades lamina propria or muscularis mucosae
T1b
Tumor invades submucosa
T2
Tumor invades muscularis propria
T3
Tumor invades adventitia
T4
Tumor invades adjacent structures
T4a
Resectable tumor invading pleura, pericardium, or diaphragm
T4b
Unresectable tumor invading other adjacent structures, such as aorta, vertebral body, trachea, etc.
Regional lymph nodes (N)c
NX
Regional lymph nodes cannot be assessed
N0
No regional lymph node metastasis
N1
Regional lymph node metastases involving 1–2 nodes
N2
Regional lymph node metastases involving 3–6 nodes
N3
Regional lymph node metastases involving 7 or more nodes
Distant metastasis (M)
M0
No distant metastasis
M1
Distant metastasis
Table 15.2
AJCC anatomic stage and prognostic groups for squamous cell carcinoma and adenocarcinoma of the esophagus
Squamous cell carcinomaa | |||||
|---|---|---|---|---|---|
Stage | T category | N category | M category | G category | Location |
0 | Is (HGD) | 0 | 0 | 1 | Any |
IA | 1 | 0 | 0 | 1, X | Any |
IB | 1 | 0 | 0 | 2–3 | Any |
2–3 | 0 | 0 | 1, X | Lower, X | |
IIA | 2–3 | 0 | 0 | 1, X | Upper, middle |
2–3 | 0 | 0 | 2–3 | Lower, X | |
IIB | 2–3 | 0 | 0 | 2–3 | Upper, middle |
1–2 | 1 | 0 | Any | Any | |
IIIA | 1–2 | 2 | 0 | Any | Any |
3 | 1 | 0 | Any | Any | |
4a | 0 | 0 | Any | Any | |
IIIB | 3 | 2 | 0 | Any | Any |
IIIC | 4a | 1–2 | 0 | Any | Any |
4b | Any | 0 | Any | Any | |
Any | N3 | 0 | Any | Any | |
IV | Any | Any | 1 | Any | Any |
Adenocarcinoma | ||||
|---|---|---|---|---|
Stage | T category | N category | M category | G category |
0 | Is (HGD) | 0 | 0 | 1 |
IA | 1 | 0 | 0 | 1–2, X |
IB | 1 | 0 | 0 | 3 |
2 | 0 | 0 | 1–2, X | |
IIA | 2 | 0 | 0 | 3 |
IIB | 3 | 0 | 0 | Any |
1–2 | 1 | 0 | Any | |
IIIA | 1–2 | 2 | 0 | Any |
3 | 1 | 0 | Any | |
4a | 0 | 0 | Any | |
IIIB | 3 | 2 | 0 | Any |
IIIC | 4a | 1–2 | 0 | Any |
4b | Any | 0 | Any | |
Any | N3 | 0 | Any | |
IV | Any | Any | 1 | Any |
In the new 2010 staging system, Tis is redefined and T4 disease is subclassified. In particular, Tis refers to high-grade dysplasia and includes all noninvasive neoplastic epithelia, previously called carcinoma in situ. T4 is subclassified according to the involvement of adjacent structures by disease. For example, the esophagus lies in close proximity to the pleura, peritoneum, pericardium, and diaphragm. Esophageal cancer that invades these structures (T4a) may be resectable. Esophageal cancer that invades the aorta, carotid vessels, azygos vein, trachea, left main bronchus, and vertebral bodies (T4b) is often unresectable. The location of the primary tumor within the esophagus has particular relevance to the draining lymph node stations for that area. Regional lymph nodes have been redefined as lymph nodes that extend from the periesophageal cervical region to the celiac region. N staging is now subclassified according to the number of regional lymph nodes with metastasis (positive nodes). N1 implies 1–2 positive nodes, N2 implies 3–6 positive nodes, and N3 implies 7 or more positive nodes. Each additional positive node is thought to increase risk. M staging has been redefined as well. The previous M1a and M1b subclassifications are no longer used. Sites of distant metastasis include disease in nonregional lymph nodes or at sites that are not in direct continuity with the esophagus and are either absent (M0) or present (M1).
The treatment of patients with esophageal cancer often involves a multimodality approach. Surgical intervention, although potentially curative, is invasive and has associated morbidity and mortality (Fig. 15.1). Estimates of operative mortality depend on the age and comorbidities of the patient population under investigation but may be up to 10% with perioperative complications in 50% of cases [21]. Over recent years, there has been increasing utilization of neoadjuvant chemotherapy and radiation in patients with potentially resectable esophageal cancer. The aim of such therapy is to eradicate metastatic and micrometastatic disease and to downstage the primary tumor, improving surgical resection and overall survival. Efficacy of long-term treatment depends on accurate preoperative staging. Controversies persist regarding the optimal surgical approach, extent of nodal resection, and precise role of chemotherapy and radiation. In general, patients with advanced esophageal cancer may benefit from referral to a specialized center with experience in multimodality therapy [3, 4].
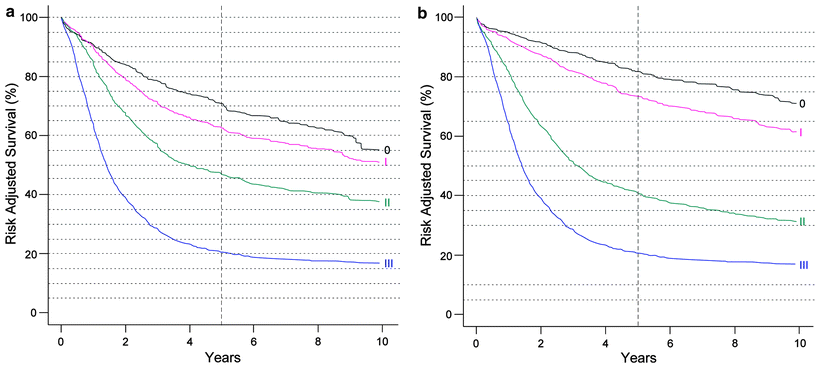

Fig. 15.1
(a) Survival after esophagectomy only for squamous cell carcinoma stratified by stage groupings, based on worldwide esophageal cancer collaboration (WECC) data. Condensed stage groupings. (b) Survival after esophagectomy only for adenocarcinoma stratified by stage groupings, based on worldwide esophageal cancer collaboration (WECC) data. Condensed stage groupings. (Used with the permission of the American Joint Committee on Cancer (AJCC), Chicago, IL. The original source for this material is the AJCC Cancer Staging Manual, Seventh Edition (2010) published by Springer Science and Business Media LLC. http://www.springer.com)
Imaging
There are several imaging modalities used in the evaluation of patients with esophageal cancer including a barium-swallow examination, endoscopy, EUS, CT, and PET. An esophagogram or barium-swallow examination may be the first study requested in the evaluation of a patient with nonspecific symptoms suggesting esophageal disease and may show a stricture or ulceration in a patient with esophageal cancer [9].
Endoscopy is often suggested for the anatomic evaluation of localized esophageal cancer. It is readily accessible, provides histologic confirmation of tumor type, and may be therapeutic in that small tumors can be resected at the time of the procedure [22]. EUS combines high-frequency ultrasound with endoscopy and has the ability to view the esophagus and delineate the layers of the esophageal wall. It has been shown to affect preoperative management and may be superior to endoscopy, CT, and PET for the evaluation of the primary tumor and local lymph nodes. Unfortunately, EUS cannot detect distant metastases and is limited when esophageal obstruction impedes passage of the endoscope [23–25].
CT may provide helpful anatomic information in patients with esophageal cancer and may be used to show the site of primary disease when EUS is indeterminate. Furthermore, CT may be used to evaluate lymph node disease, although inflammation can be difficult to distinguish from sites of neoplastic disease and micrometastasis is undetectable. The strength of CT, however, lies in its ability to generate high-resolution anatomic images of a large field of view. For this reason, it is the imaging modality of choice to define vascular supply and direct surgical resection approach. CT is also useful in the evaluation of metastatic disease [3, 4].
The Role of PET
[18F]FDG-PET provides insight into tumor metabolism and may be used to assess metabolic activity of the site of primary esophageal cancer and metastatic disease. Although PET may be performed without CT, the advent of PET with integrated CT allows for direct comparison of metabolic information and anatomy (Fig. 15.2). Since 2001, when the first clinical PET/CT system became operational, imaging protocols have been evolving. However, to date, there is no standardization. The use of iodinated contrast, CT technique, and optimal radiotracer dose as well as uptake period remains controversial. Irrespective of the above considerations, the integration of PET with CT has certainly led to an improvement in diagnostic accuracy, and [18F]FDG-PET/CT is becoming an increasingly popular tool to obtain metabolic insight in oncology patients.
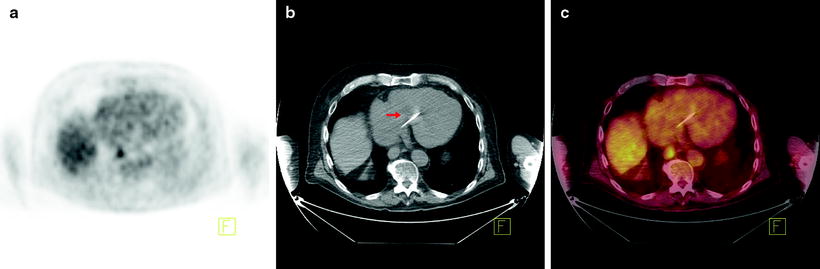

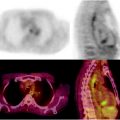
Fig. 15.2
Additional utility of integrated [18F]FDG-PET/CT in the detection of esophageal cancer. Seventy-six-year-old man with newly diagnosed invasive esophageal adenocarcinoma of the distal esophagus on endoscopic biopsy, for staging. (a) Axial PET image shows focal uptake in the region of the esophagus. (b) Axial CT image shows essentially normal esophagus. (c) Fused axial PET/CT image confirms focal uptake in the esophagus at the site of biopsy proven adenocarcinoma. Incidental note is made of a pacemaker lead on the CT image (arrow)
[18F]FDG-PET and T Stage
PET typically generates low spatial resolution images of a large field of view. The sensitivity of this modality for the detection of small lesions, mucinous tumors, or low cellularity disease is low. In patients with esophageal cancer, [18F]FDG-PET is not used to detect early stage disease or to evaluate the depth of tumor invasion into the esophageal wall. In a recent study of a series of 149 patients with thoracic esophageal carcinoma, [18F]FDG-PET detected the primary lesion in 43% of T1 tumors. Sensitivity was significantly better for pT1b disease (involving the submucosa) at 61%, compared to 18% for T1a disease (confined to the muscularis mucosa). PET positivity increased with increasing levels of tumor invasion being 90% at T2, 87% at T3, and 100% at T4 [26]. Indeed, [18F]FDG-PET and PET/CT are inadequate for assessing the depth of tumor penetration within the mucosal wall of the esophagus and cannot distinguish adequately between carcinoma in situ and invasive disease.
In addition, [18F]FDG avidity on [18F]FDG-PET scans should be taken in context due to the small but real rate of false-positive scans. Specifically, areas of increased [18F]FDG uptake within the esophagus may have an alternate cause such as chemotherapy- or radiation-induced esophagitis, candida, or other benign etiologies [27–30]. [18F]FDG-PET lacks the specificity to differentiate between these conditions, underscoring the inadequacy of this approach. In an attempt to overcome this particular problem, a study comparing the characteristics of benign causes of increased [18F]FDG uptake with those of premalignant disease and early cancers demonstrated that early malignant lesions had higher intensity, focality, and eccentricity when compared to benign lesions. A scoring system combining these elements was proposed to differentiate between these two etiologies [31]; however, this has not been widely adopted.
[18F]FDG-PET and N Stage
Initial reports of [18F]FDG-PET showed promise due to apparent increased sensitivity for the detection of lymph node metastasis when compared to CT [32]. However, this may have been due to the use of outdated CT technology and techniques, and this initial promise with respect to increased sensitivity has not been sustained in well-designed prospective studies. Progress in the development of both CT and PET imaging has led to improvements in the diagnostic accuracy of both modalities. A recent study comparing thin slice CT to [18F]FDG-PET/CT in the detection of subclinical lymph node metastasis in patients with operable squamous cell carcinoma demonstrated the superiority of CT for the detection of disease at all lymph node stations, with the caveat that sensitivity appeared to decrease from the cervical area (100%) to the abdominal area (22%). Specificity was high for both CT and [18F]FDG-PET in the cervical and abdominal lymph node basins, with superior specificity for PET demonstrated only within the mediastinum [33].
The limited spatial resolution of PET may lead to difficulties due to the fact that uptake within lymph nodes close to the primary tumor may be difficult to distinguish from the tumor itself. Fusion PET/CT and correlation with metabolic- and tumor-related parameters may offer superior sensitivity for the detection of nodal disease. A 2009 study by Roedl et al. compared fusion [18F]FDG-PET/CT with PET viewed side by side with CT images, in addition to axial tumor area, tumor diameter, and SUV uptake. Fusion [18F]FDG-PET/CT was more sensitive and more specific for the detection of lymph node metastasis at 70% versus 62% and 95% versus 91%, respectively. Sensitivity and specificity were increased to 87% and 85% by the addition of tumor diameter measurements. When qualitative visual analysis was added to quantitative tumor dimension measurement in addition to PET/CT, the sensitivity yielded was 96% and the specificity 95% [34].
Dual-time-point [18F]FDG-PET may assist in the differentiation between benign and malignant lesions and may also improve the accuracy of detecting lymph node metastasis in esophageal cancer. Small malignant lesions and malignant lymph nodes show an increase in SUV uptake over time, whereas benign disease does not and shows an early peak only. An improvement in diagnostic accuracy from 83% to 91% was seen with dual time imaging of squamous cell carcinomas of the thoracic esophagus. In addition, false-positive uptake in the lung hilum due to inflammatory processes was distinguished from malignant disease in 19/42 (45%) of patients using this method [35].
[18F]FDG-PET and M Stage
The main role of [18F]FDG-PET is in the detection of metastatic disease (Fig. 15.3). Most patients with esophageal cancer present with advanced disease, and the ability to accurately detect metastases may have a profound impact on management. Indeed, the National Comprehensive Cancer Network recommends the use of [18F]FDG-PET in staging patients lacking M1 disease on other imaging. Findings on CT and PET may be complementary in the detection of metastases to the lungs, liver, and lymph nodes. Atypical sites of metastases including skeletal muscle, subcutaneous soft tissue, or pleura are often much more difficult to identify on anatomic imaging alone (Fig. 15.4). A 2008 meta-analysis reported that [18F]FDG-PET had 71% sensitivity and 93% specificity for distant metastases compared with 52% and 91% for CT, respectively [36]. [18F]FDG-PET/CT may also incidentally identify significant medical findings. For example, Fig. 15.5 shows selected images from an [18F]FDG-PET/CT study done at the time of esophageal cancer staging. Diffuse uptake in the bowel was consistent with the clinical history of Metformin use in a diabetic patient. A horseshoe kidney was also incidentally discovered. Figure 15.6 illustrates a renal cell carcinoma that was incidentally discovered at the time of [18F]FDG-PET/CT staging for esophageal carcinoma. A recent multicenter prospective study from Australia reported that of 129 patients, [18F]FDG-PET found additional sites of disease in 41% and led to a significant change in management in 38% of patients [37]. Finally, [18F]FDG-PET may have a cost-effective impact on patient care. For example, Wallace et al. studied the cost-effectiveness of various staging options for patients with esophageal cancer. Using a decision-analysis model with cost derivation from the surveillance epidemiology and end results (SEER)-Medicare-linked database, the combination of [18F]FDG-PET and EUS-FNA was found to be more cost-effective than CT and EUS-FNA. Integrated [18F]FDG-PET/CT was not available in this study [38].
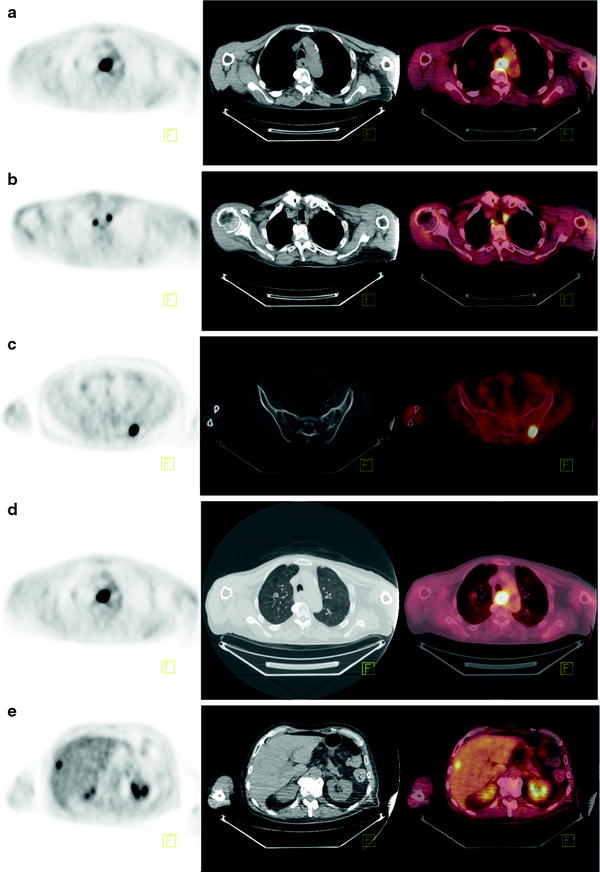
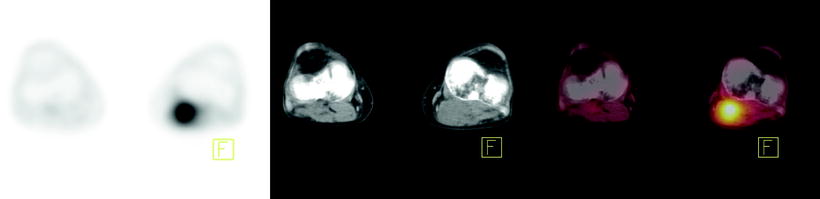
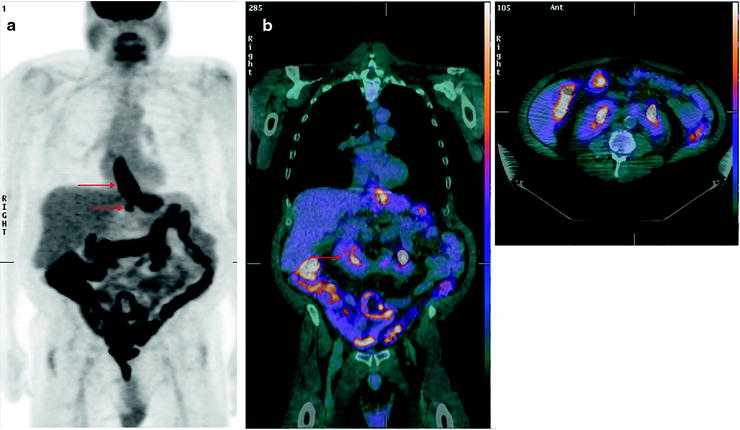
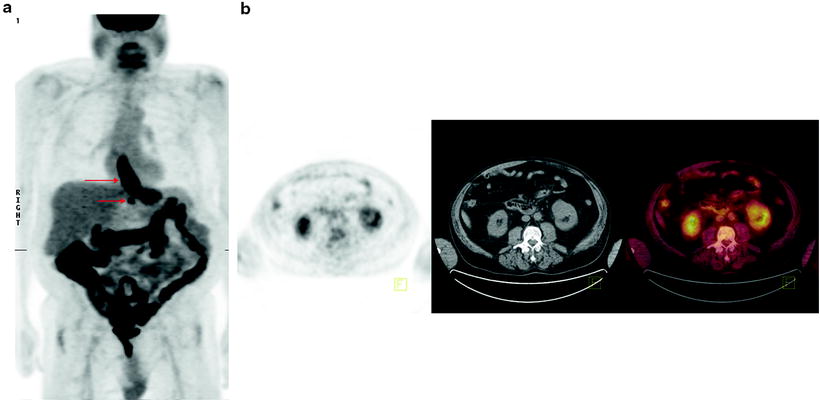

Fig. 15.3
[18F]FDG-PET, CT, and fused PET/CT images as tools to evaluate distant metastasis in esophageal cancer. Eighty-three-year-old man with poorly differentiated squamous cell carcinoma of the mid-esophagus, for staging. (a) Axial PET, CT, and fused PET/CT images showing [18F]FDG-avid circumferential thickening of the esophageal wall at the site of primary disease. (b) Axial PET, CT, and fused PET/CT images showing [18F]FDG-avid lymphadenopathy. (c) Axial PET, CT, and fused PET/CT images showing [18F]FDG-avid osseous metastasis. (d) Axial PET, CT, and fused PET/CT images showing [18F]FDG-avid pulmonary metastasis. (e) Axial PET, CT, and fused PET/CT images showing [18F]FDG-avid liver metastasis

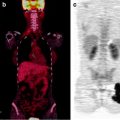
Fig. 15.4
[18F]FDG-PET, CT, and fused PET/CT images as tools to evaluate atypical sites of metastasis in esophageal cancer. Forty-year-old man with metastatic esophageal cancer. Axial PET, CT, and fused PET/CT images showing [18F]FDG-avid disease in the left popliteal fossa

Fig. 15.5
Significant incidental findings identified on [18F]FDG-PET/CT. Fifty-seven-year-old man with esophageal cancer, for staging. (a) MIP showing [18F]FDG-avid disease in the distal esophagus at the site of known esophageal carcinoma (upper arrow), [18F]FDG-avid adenopathy (lower arrow), and diffuse uptake in bowel consistent with Metformin use. (b) Coronal and axial fused PET/CT images showing a horseshoe kidney (arrow)

Fig. 15.6
Significant incidental finding identified on [18F]FDG-PET/CT. Sixty-three-year-old man with esophageal cancer, for staging. (a) Axial PET, CT, and fused PET/CT images showing [18F]FDG-avid disease in the distal esophagus at the site of known esophageal carcinoma. (b) Axial PET, CT, and fused PET/CT images showing incidental left renal mass proven to be renal cell carcinoma on biopsy
Research into the use of [18F]FDG-PET is ongoing. In particular, PET may be used to assess therapeutic response and serve as a prognostic factor for disease outcome.
Is [18F]FDG-PET Predictive of Survival in Esophageal Cancer?
Several studies have reported a relationship between [18F]FDG SUVmax and survival. In a meta-analysis of disease-free and overall survival, the hazard ratio for disease recurrence and death was 2.52 and 1.86, respectively, for those with a higher than median SUV [39]. This correlation with SUVmax and survival may hold true even for those with apparently early stage disease [40]. There is no agreement if SUVmax can be an independent risk factor. In fact, it is also often significantly correlated with pathological stage, acting as a potential confounder. Although in some studies it did not emerge as an independent risk factor [41, 42], in a large retrospective study of patients with operable esophageal cancer (mainly squamous cell carcinoma), the peak SUV was independently and significantly associated with overall survival on multivariate analysis. Five-year survival for those with a peak SUV ≥4.5 was 47% when compared to those with peak SUV <4.5 which was 76% [43].
On the other hand, other authors did not find an association between survival for patients with a high or a low SUVmax. In a recent retrospective study in patients with adenocarcinoma of the distal esophagus or gastroesophageal junction who underwent chemoradiation as a primary treatment, Rizk et al. did not observe a worse survival in patients with a high initial SUVmax. Nevertheless, these patients showed a superior response to chemoradiation acting as an equalizing factor with respect to survival [44]. Altogether, these data suggest that high SUVmax is most likely to be associated with increased tumor stage and extent. However, whether SUVmax can be an independent predictor of patient outcome is not sufficiently validated.
Does SUV Predict Response to Chemotherapy?
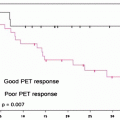
There have been many studies performed in an effort to examine whether change in SUV of the primary tumor following chemotherapy or chemoradiotherapy is useful in determining response to the intervening therapy. A large proportion of studies have been prospective, but are limited in their scope of analysis to some extent by small numbers. Each study evaluated a different treatment regimen. Most studies use pathological response as the gold standard for evaluation of chemotherapy efficacy [45]. The primary usefulness of a change in [18F]FDG uptake from baseline as a marker for response to chemotherapy and subsequently survival is that this information is available early in the treatment plan and thus could potentially be used in order to guide future management.
Several studies reported a reduction in SUV (30–60%) of responders when compared to nonresponders. Response was significantly associated with survival (60–67% vs. 37–38%) [46–49] (Fig. 15.7). In the MUNICON trial, metabolic nonresponders had an event-free survival of a median 14.1 months compared to 29.7 months in metabolic responders. However, metabolic responders who did not have a pathological response had a comparable survival with those who were metabolic nonresponders, implying that metabolic response was necessary but not sufficient for improved survival [50].
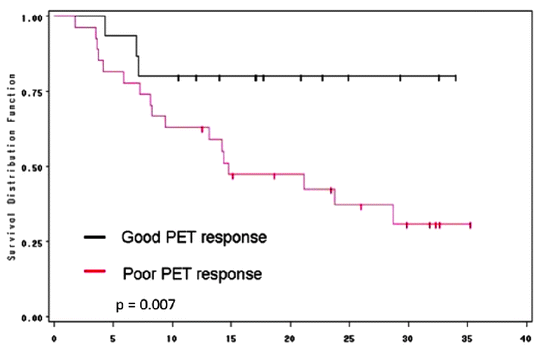

Fig. 15.7
Correlation between [18F]FDG-SUV drop following chemotherapy and patient outcome in patients receiving preoperative chemotherapy with irinotecan and cisplatin
In a cross-trial comparison [48, 50] between metabolic nonresponder patients who continued chemotherapy and those who proceeded directly to surgery, survival in both groups was similar, thus suggesting that patient survival was unaffected (either adversely or positively) by continuing with ineffective chemotherapy. These results have led to an ongoing clinical trial in which failure to respond to initial induction chemotherapy with a reduction in SUV on [18F]FDG-PET is followed by introduction of a salvage regimen of non-cross-resistant chemotherapy in an effort to improve outcome (NCT00737438 on clinicaltrials.gov; Memorial Sloan-Kettering study, IRB 08-081).
Moreover, there have been a number of negative studies with respect to correlation of response on [18F]FDG-PET with pathological response post-induction chemotherapy and/or chemoradiation [51–54]. Differences in the timing of PET evaluation, study size, categories of response, and the use of pathological stage as a surrogate for response rather than a formal methodology could explain discordant results. These studies suggest however that the usefulness of [18F]FDG-PET in response assessment remains investigational at this time, but a potentially promising modality to begin “individualized” care for patients with upper GI malignancies (namely esophageal and gastric adenocarcinoma).
It should be noted that the response of PET to chemotherapy when compared with that of CT may lead to clinical confusion, such as when a lesion improves by PET criteria, but fails to shrink or may even enlarge slightly by traditional RECIST criteria [55]. Recently proposed guidelines for response assessment in solid tumors suggest that [18F]FDG-PET progression be defined as standardized uptake value peak connected by lean body man (SUL) increase of >30% in a single hottest tumor lesion or development of new metabolically active lesions suspicious for malignancy, whereas response be defined as a decline in SUL peak of >30% [56]. Such a guideline would seem to be a good starting point for evaluation of [18F]FDG-PET response across many solid tumor malignancies and needs prospective validation.
[18F]FDG-PET for the Detection of Esophageal Cancer Recurrence
The accuracy of CT and MRI for the detection of recurrent disease, particularly within the area of the initial primary, may be decreased by postsurgical- or postchemoradiation-related changes such as fibrosis, edema, and inflammation. For this reason, several studies have examined the utility of [18F]FDG-PET for the detection of recurrent esophageal cancer. Guo et al. recently demonstrated excellent sensitivity of [18F]FDG-PET at local, regional, and distant sites of metastases (96.9%, 85.9%, and 90.5%, respectively), but lower specificity for locoregional recurrence (50%, 92.2%, and 89.9%, respectively) [57]. Moreover, a French study examined the routine use of [18F]FDG-PET in the prospective follow-up of resected esophageal cancer patients [58]. This study demonstrated that for the detection of locoregional recurrence, PET had a higher sensitivity, slightly lower specificity, and a superior accuracy than CT (100% vs. 65%, 85% vs. 91%%, and 91% vs. 81%, respectively). PET was also superior to CT in the detection of local metastasis leading to a 100% negative predictive value. As this recently published study is the first examining the prospective use of [18F]FDG-PET to detect recurrence in asymptomatic patients, it is too early to comment on whether changes in management based on this strategy will lead to improvements in patient outcomes.
Comparison of [18F]FDG to Other PET Tracers in the Diagnosis and Management of Esophageal Cancer
[18F]FDG is not a tumor-specific radiotracer, and this leads to the drawback of false-positive uptake in areas of inflammation or infection. Although other PET tracers have been proposed, these remain in the field of research. For example, 18F-FLT (3-deoxy-3-fluorothymidine) is trapped intracellularly following phosphorylation by thymidine kinase 1 (TK1) and is thought to serve as a marker of cell proliferation [59]. However, a study by van Westreenan et al. compared the efficacy of 18F-FLT versus [18F]FDG in the detection of esophageal cancer and suggested [18F]FDG was preferable to 18F-FLT18. [11C]choline-PET may have superior sensitivity in the detection of primary tumors and nodal metastases in the mediastinum (at 94% and 88%, respectively) [60], although results in the literature are mixed [61].
[18F]FDG-PET/CT Imaging of the Small Bowel and Associated Benign and Malignant Diseases
The normal pattern of [18F]FDG distribution includes the gastrointestinal tract, and tracer uptake can be seen in the esophagus, stomach, small intestine, and colon. The pattern of uptake seen on [18F]FDG-PET is often diffuse and usually physiologic [62]. However, a focal area of increased [18F]FDG uptake may be equivocal or suggestive of a malignancy [63]. When evaluating for neoplastic disease involving the small and large bowel with [18F]FDG-PET/CT, one must first take into account spurious physiologic [18F]FDG uptake in the gastrointestinal tract related to a combination of uptake by smooth muscles (secondary to normal peristalsis), swallowed secretions, gastrointestinal lymphoid tissue, and excreted radiotracer concentrated within the bowel lumen [64]. Low-grade uptake is a normal finding frequently seen throughout the small bowel, while diffuse and/or mild focal uptake is commonly associated with normal colon [62].
Lymphomatous Bowel Involvement
[18F]FDG-PET is highly sensitive and specific for staging and for the evaluation of treatment response of nodal as well as extranodal sites of malignant lymphoma. The gastrointestinal tract is the most frequently involved extranodal site of non-Hodgkin’s lymphoma (NHL). Gastrointestinal NHL accounts for 4–20% of all NHLs and 30–40% of all extranodal cases [65]. The disease could either primarily involve the gastrointestinal tract or present as disseminated nodal disease with secondary gastrointestinal involvement. Lymphomas can involve any part of the gastrointestinal tract, from the mouth to the rectum. After the stomach, the small intestine and colon are the most frequently involved gastrointestinal sites [66]. Approximately half to two-thirds of gastrointestinal NHLs are diffused large B cell lymphomas [67]. Primary involvement of the bowel by Hodgkin’s disease has not been documented [68]. Clinically, almost half of the patients with small bowel lymphoma present with an acute abdominal emergency [69].
Imaging
CT is the most commonly used imaging modality to evaluate and manage lymphoma. With lymphoma involving the bowel, the most commonly encountered features on the corresponding CT portion of a [18F]FDG-PET/CT evaluation are bowel wall thickening and/or focal masses (Figs. 15.8 and 15.9). Other CT features associated with bowel lymphomas are aneurysmal dilatation of affected bowel loops and bowel obstruction.
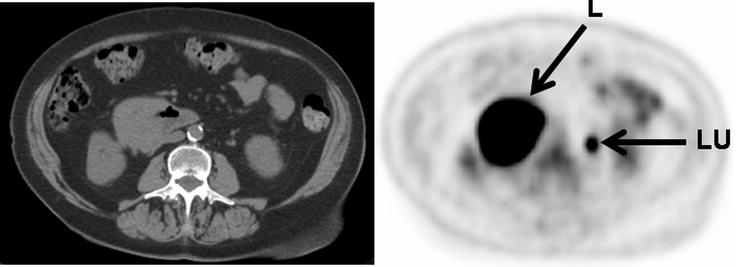
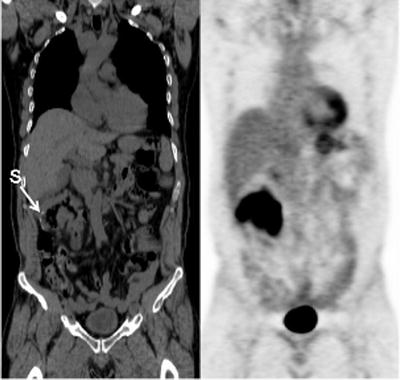

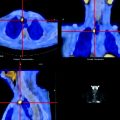
Fig. 15.8
Intensely [18F]FDG-avid lymphoma presenting as a mass involving the third portion of the duodenum (L: lymphoma, LU: activity in left ureter)

Fig. 15.9
Recurrent intensely [18F]FDG-avid lymphoma presenting as diffuse bowel wall thickening involving the hepatic flexure of the colon just distal to an anastomotic suture line (S: suture line)
Following treatment, most patients with gastrointestinal lymphomatous involvement have residual soft tissue masses [70]. On CT, a response to treatment correlates with a decrease in the pretreatment size of a lymphomatous mass. However, even with clinical response, a decrease in size of a lymphomatous mass may take a relatively long time to occur. Furthermore, a significant decrease in size may not occur posttreatment if there is superimposed fibrosis, necrosis, and/or inflammation [71]. Therefore, CT may be unable to differentiate between viable tumor and inactive (fibrous) tissue following treatment.
Functional imaging has had a major impact on the evaluation and management of patients with Hodgkin’s disease and non-Hodgkin’s lymphoma. Prior to [18F]FDG-PET, 67Ga-citrate scintigraphy was used to stage patients with lymphoma, evaluate response to therapy early after initiation of treatment, assess the significance of a residual mass, and serve as a prognostic factor. However, [18F]FDG-PET has now replaced 67Ga-citrate scintigraphy in the evaluation of patients with lymphoma. [18F]FDG-PET/CT is used not only to detect lymphomatous bowel involvement but also to differentiate between viable residual and recurrent disease in the bowel versus scar tissue after therapy (Fig. 15.10). As [18F]FDG-PET/CT evaluation is based on tissue metabolism rather than changes in physical size, post-therapeutic changes are often detected earlier than on conventional CT imaging. There is intense [18F]FDG uptake in viable lymphoma and relatively low uptake in indolent fibrotic tissue [72]. Kumar et al. reported that [18F]FDG-PET had a positive predictive value (PPV) of 100% for the evaluation of residual or recurrent lymphoma after treatment. All patients with positive post-therapy [18F]FDG-PET relapsed within 1 year. However, only one patient (7.7%) with a negative [18F]FDG-PET result after therapy relapsed [65]. [18F]FDG-PET/CT may, however, have limited sensitivity to detect minimal residual lymphoma [73].
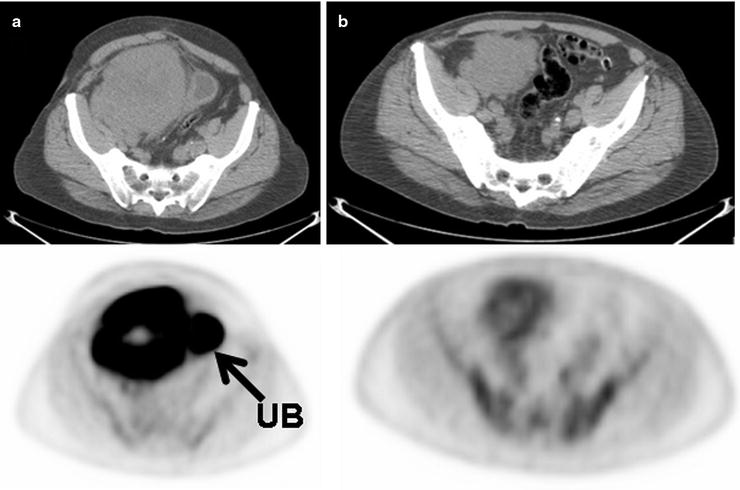

Fig. 15.10
Large B cell lymphoma mass involving the cecum before treatment (a) and mid-cycle of chemotherapy (b) shows some interval decrease in size of the mass but a significant decrease in [18F]FDG uptake. The lack of [18F]FDG uptake greater than that of blood pool levels in the mass following the start of treatment is a good prognostic sign and suggests lack of significant residual viable tumor. (UB: excreted [18F]FDG uptake in urinary bladder)
Other less common forms of NHL commonly arise in or involve the gastrointestinal tract and can be evaluated and followed by [18F]FDG-PET/CT. In its sporadic form, Burkitt’s lymphoma, a fast-growing high-grade B cell neoplasm involving a characteristic translocation between the long arms of chromosomes 8 and 14, often involves the bowel, namely, the terminal ileum and/or cecum. [18F]FDG-PET/CT has been shown to be sensitive for the detection of viable disease in Burkitt’s lymphoma (Fig. 15.11). Affected areas demonstrate a high degree of uptake that resolves upon successful implementation of treatment [74].
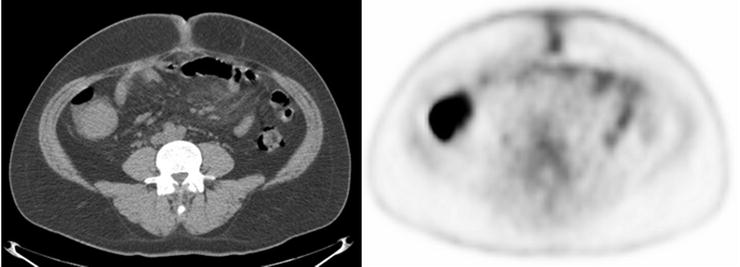

Fig. 15.11
Burkitt’s lymphoma presenting as an intensely [18F]FDG-avid cecal mass
Extranodal marginal zone lymphoma, also known as mucosal-associated lymphoid tissue (MALT) lymphoma, is a low-grade lymphoma and the third most common type of NHL. It can involve mucosal surfaces anywhere in the body and commonly presents in the gastrointestinal tract, with the stomach being the most common site of involvement (Fig. 15.12). The reliability of [18F]FDG-PET/CT for the evaluation of MALT lymphoma is under some debate. Initial studies by Hoffman et al. found that [18F]FDG-PET/CT was unreliable for detecting MALT lymphoma, while a later study by Beal et al. demonstrated [18F]FDG avidity in 80% of cases [75, 76]. Hoffman et al. then described that the histologic subtype of the MALT lymphoma may influence whether a neoplasm is significantly [18F]FDG-avid, with much higher PET sensitivities seen in plasmacytically differentiated MALT (83%) compared to typical MALT (20%) [77].
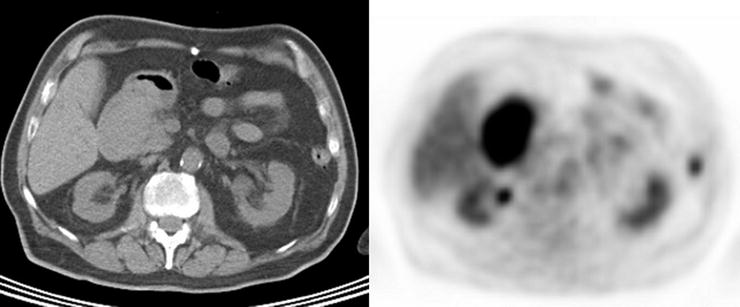

Fig. 15.12
An example of intensely [18F]FDG-avid mucosal-associated lymphoid tissue (MALT) lymphoma presenting as a duodenal mass
Colonic Polyps
There are several types of benign colonic polyps including adenomas, hyperplastic polyps, juvenile polyps, and inflammatory polyps. Colonic adenomas have a potential for malignant transformation, and evolving colorectal carcinoma can progress insidiously in an asymptomatic patient. It is an accepted medical practice to remove adenomatous colonic polyps when they are found.
Adenomas and hyperplastic polyps are the most prevalent types of colonic polyps, but only adenomas have the potential for malignant transformation (Fig. 15.13 and Table 15.3). Prior studies have demonstrated no significant [18F]FDG uptake in hyperplastic polyps [78]. However, adenomatous polyps have been shown to accumulate [18F]FDG and are detected incidentally on PET (Fig. 15.14). Kamel et al. described that up to 39% of incidentally detected [18F]FDG-avid lesions in the gastrointestinal tract on PET/CT were colonic adenomas [79]. Larger colonic adenomas (10 mm or greater) have a higher tendency to be detected by PET than smaller polyps, a finding most likely related to the partial-volume effect as it relates to the spatial resolution of PET scanners [80]. However, as the likelihood of malignant transformation of adenomas increases with their size, “higher-risk” polyp uptake is more likely to be described incidentally on [18F]FDG-PET reports. Whereas most literature series report 10 mm as the lower limit of adenoma size for PET detection, with the improved resolution of PET scanners, small to intermediate polyps (4–6 mm) are detected, although at a much lower rate than larger polyps [81]. In addition to the potential small size of adenomas, non-pathologic colonic [18F]FDG uptake from lymphoid tissue, muscular contraction, inflammation, sloughed cells, errors in attenuation correction, and artifact caused by collapsed bowel loops may confound matters with false-positive results.
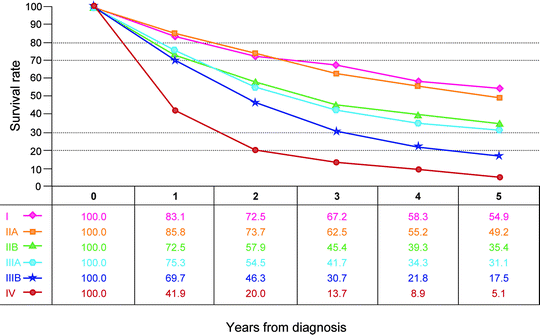
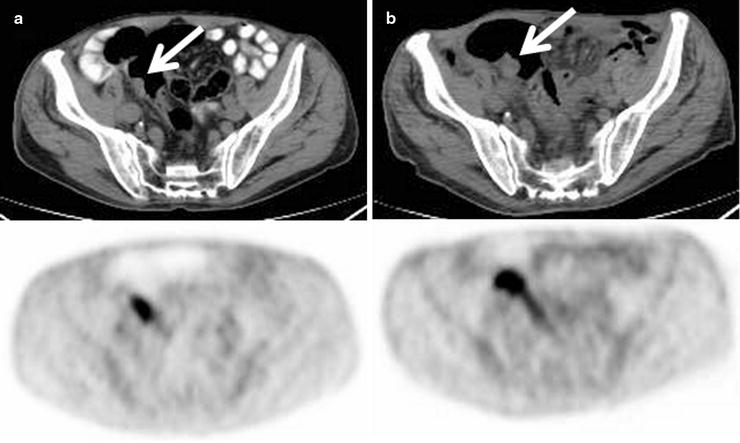

Fig. 15.13
Observed survival rates for 3,086 cases with adenocarcinoma of the small intestine. Data from the National Cancer Data Base (Commission on Cancer of the American College of Surgeons and the American Cancer Society) diagnosed in years 1998–2002. Stage I includes 328; Stage IIA, 685; Stage IIB, 304; Stage IIIA, 715; Stage IIIB, 328; and Stage IV, 726. (Used with the permission of the American Joint Committee on Cancer (AJCC), Chicago, IL. The original source for this material is the AJCC Cancer Staging Manual, Seventh Edition (2010) published by Springer Science and Business Media LLC. http://www.springer.com)
Table 15.3
AJCC anatomic stage and prognostic groups for small bowel carcinoma
Anatomic stage | Prognostic groups | ||
|---|---|---|---|
Group | T category | N category | M category |
Stage 0 | Tis | N0 | M0 |
Stage I | T1 | N0 | M0 |
T2 | N0 | M0 | |
Stage IIA | T3 | N0 | M0 |
Stage IIB | T4 | N0 | M0 |
Stage IIIA | Any T | N1 | M0 |
Stage IIIB | Any T | N2 | M0 |
Stage IV | Any T | Any N | M1 |

Fig. 15.14
Progressive increase in size and [18F]FDG uptake associated with an adenomatous polyp (arrows) incidentally detected on PET/CT scan in a patient being treated for lymphoma (a: baseline, b: 6 months later)
Adenomas in the cecum, ascending colon, and descending colon have been observed to be more commonly detected than adenomas elsewhere in the large intestine. It has been hypothesized that decreased mobility of colonic segments increases the likelihood of detecting adenomas, as the cecum, ascending colon, and descending colon are fixed at the retroperitoneum with relatively restricted mobility [80]. False-negative activity is likely related to blurring of small [18F]FDG-avid foci from respiratory or peristaltic motion and gross shifts in bowel position over a relatively long PET scanning time [81].
Although size and mobility may play significant roles in polyp detection by PET, data suggests that a more complex relationship exists. For example, Gollub et al. described 16 adenomatous polyps larger than 1 cm which were not significantly [18F]FDG-avid [81]. The presence or absence of high-grade dysplasia within the specific adenomas is thought to play a role. Also, polyps with a flat morphology, even if larger than 10 mm, may tend to be missed on [18F]FDG-PET due to partial-volume averaging effects based on how they are oriented at the time of imaging.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree