From 1999 through 2008, most visits to the emergency departments (EDs) in the United States by adult patients (≥15 years old) were mainly for chest and abdominal pain, according to the latest National Hospital Ambulatory Medical Care Survey released in September of 2010 by the Centers for Disease Control and Prevention. In 2007 and 2008, approximately 5.5 million visits were primarily attributed to chest pain. Among the visits for chest pain, a minority was caused by acute coronary syndrome (ACS), aortic dissection, or pulmonary embolism.
Besides the introduction of multiple medical advances in the evaluation of acute chest pain in the ED, differentiating patients who need to be admitted from those who can be safely discharged poses a difficult and complex challenge for emergency medicine clinicians. Most patients who present with chest pain and who are ultimately admitted are eventually discharged without a diagnosis or with a diagnosis of a noncardiac condition. This situation represents a significant financial burden, estimated to be approximately $8 billion in the United States each year. Moreover, patients who are inappropriately discharged and who ultimately prove to have ACS have an increased mortality rate (almost double) when compared with those who are admitted. In a typical ED in the United States, approximately 2% of patients with acute myocardial infarction and 2% of those with unstable angina are inappropriately discharged; this finding exemplifies that no model exists to identify successfully all patients with medically serious chest pain.
Standard Approach in Diagnosing Acute Coronary Syndrome
ACS, which is usually caused by a ruptured atherosclerotic plaque, has different clinical presentations, ranging from unstable angina to subendocardial or transmural infarction. The most common clinical presentation of acute myocardial ischemia is chest pain, which is primarily the result of interrupted blood flow to the myocardium. Nevertheless, the clinical manifestations of chest pain are often ambiguous, and they present a major challenge in accurately differentiating ACS from other serious conditions such as pulmonary embolism and aortic dissection, as well as less serious noncardiac causes of chest pain such as pneumonia, gastroesophageal reflux, and musculoskeletal discomfort.
Since 2000, multidetector computed tomography (MDCT) has become the standard modality used to diagnose pulmonary embolism. MDCT allows routine visualization of the pulmonary arteries to the subsegmental level, with reported sensitivity and specificity ranging from 83% to 100% and 89% to 97%, respectively. MDCT has also become the gold standard for detection of suspected aortic dissection, with reported sensitivity and specificity greater than 95%. The urgent nature of arriving at a diagnosis of aortic dissection in the ED, the short time it takes for a scan to be performed, and the proximity of scanners to most EDs make MDCT highly attractive. The nonenhanced component of the scan is useful to detect the presence of intramural hematoma. Such a hematoma is apparent as a thickened aortic wall with increased radiodensity. In the absence of a dissection flap and a false lumen, an intramural hematoma may not be visible following the administration of contrast media.
In most cases, the diagnosis of ACS follows a standard approach that includes an initial evaluation phase, followed by a period of prolonged observation related to the diagnostic complexity of ACS. This observation period also prevents inappropriate discharge of patients with ACS. The initial assessment of ACS incorporates the clinical triad of symptoms, electrocardiogram (ECG), and cardiac enzyme levels (creatine kinase and troponin). The most common physical symptoms have classically been described as chest pain with exertion and pressure-like chest discomfort with radiation to the neck or arms. Nevertheless, the clinical presentation of ACS is often nonspecific, thus limiting the use of this clinical triad. As many as 20% of patients with myocardial infarction present with atypical chest pain, and another subset of patients presents with no pain at all. The second component of the initial assessment, the ECG, has limitations because approximately 20% of ECGs are normal in acute myocardial infarction, 37% in the setting of unstable angina. Classically, the ECG is diagnostic in the case of ST-segment elevation myocardial infarction and occasionally diagnostic for non–ST-segment elevation myocardial infarction, an inconsistency attributed to the sporadic nature of coronary ischemia.
Cardiac troponin has become the biomarker of choice for patients with ACS, especially in the ED; however, multiple limitations restrict biomarker application to acute myocardial infarction. The presence of biomarker elevation confirms only that myocardial injury has occurred, and myocardial injury can be seen in multiple conditions in addition to ACS, such as heart failure, myocarditis, pericarditis, pulmonary embolism, sepsis, or any other condition resulting in ischemia or inflammation of the myocardium. The sensitivity of biomarkers for acute myocardial infarction has been found to vary depending on when biomarker levels are measured; increases in sensitivity have been reported with serial measurements. The use of these biomarkers in patients with ACS is often inconclusive initially because it generally requires approximately 2 to 4 hours after the onset of symptoms for biomarker levels to be detectable.
The reported sensitivity of initial biomarkers for acute myocardial infarction is between 37% and 49%, with an increase to 79% to 93% when serial measurements are performed. Because of the initial limitations, the American College of Cardiology and the American Heart Association recommend repeat testing 8 to 12 hours after the initial levels are measured. This recommendation justifies the current period of observation used to improve diagnostic sensitivity and overall detection of ACS, although this approach comes at a high financial burden to society. These limitations highlight the need for approaches that can provide high diagnostic sensitivity while reducing the number of unnecessary admissions and the exposure of risky procedures to patients without ACS. The use of noninvasive cardiac imaging has the potential to achieve these goals.
Patient Stratification in the Evaluation of Acute Coronary Syndrome
The Thrombolysis in Myocardial Infarction (TIMI) risk score was introduced to stratify patients more accurately based on risk of cardiac events. Noninvasive imaging plays different roles depending on the likelihood of ACS. From this perspective, patients can be categorized into three groups.
The first group consists of patients who present with clear evidence of ACS by physical examination, ECG, and cardiac biomarkers. According to the guidelines of the American College of Cardiology and the American Heart Association, these patients are usually admitted for further interventions such as coronary angiography and, if necessary, reperfusion invasive therapies. Noninvasive imaging has no role in the immediate care of these patients.
The second group includes patients who present with clearly benign symptoms or other noncardiac conditions that do not require admission. These patients can usually be safely discharged from the ED without any further cardiac imaging assessment.
The third group, previously labeled the indeterminate group, is the most difficult group of patients to treat. These patients, usually in their fourth to sixth decades of life, present with an atypical clinical conglomeration of symptoms and laboratory results that do not fit any specific traditional risk factor criteria: atypical chest pain that deviates from the classic ischemic type, either normal or nonspecific ECG changes (nonspecific T-wave changes), and normal cardiac biomarker levels. These patients fall between the two previously described groups with risk factors that are low to intermediate for ACS, and they are at high risk of inappropriate discharge from the ED and possible future complications. In the ED, these patients are usually evaluated by a cardiologist. In many cases, they undergo noninvasive evaluation with conventional techniques including exercise treadmill testing and noninvasive cardiac imaging such as single photon emission computed tomography (SPECT) myocardial perfusion, and echocardiography. More recently, coronary computed tomography angiography (CTA) has been developed as a tool to help in the triage of these patients by providing a high negative predictive value for the exclusion of ACS.
Role of Noninvasive Imaging in the Conventional Assessment of Acute Coronary Syndrome
The pathophysiologic process from myocardial ischemia to infarction is dynamic and involves multiple phases at the vascular level. This process can be associated either with normal findings or with perfusion mismatches that lead to abnormalities of diastolic and systolic myocardial function. The goal of imaging is to identify processes that occur upstream to myocardial infarction, thus providing a mechanism for prompt intervention either by modification of the patient’s dietary habits or by the use of pharmacologic agents. To date, the most frequent noninvasive imaging modalities used for the evaluation of possible ACS are chest radiography, exercise tolerance testing (ETT), transthoracic echocardiography, and radionuclide perfusion imaging, as discussed in the following subsections. Echocardiography and radionuclide perfusion play important roles as noninvasive imaging modalities currently used for the stratification of risk in patients subsequent to observation and a negative serial biomarker evaluation. These modalities are important adjuncts in the identification of high-risk patients with coronary disease because cardiac biomarker findings can be negative in the absence of myocardial death.
Chest Radiograph
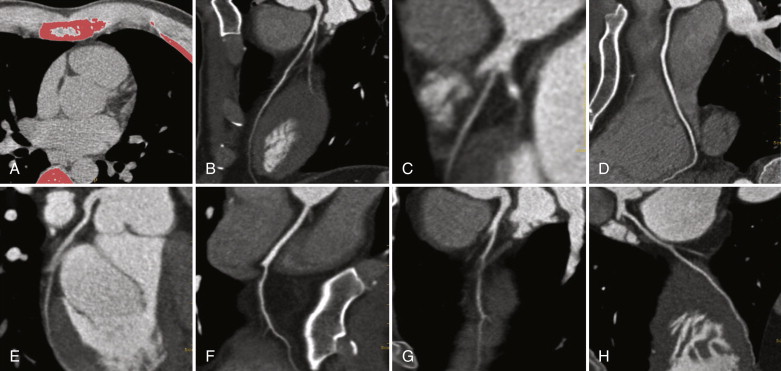
In the ED, the chest radiograph is most commonly the first imaging modality used in the evaluation of patients presenting with chest pain. The attractiveness of this technique stems from its ability to exclude major noncardiac causes of chest pain (e.g., pneumonia, pneumothorax, or rib fracture) rapidly. The chest radiograph provides a global assessment of chest anatomy and any life-threatening conditions that require prompt intervention. The presence of myocardial calcification suggests prior myocardial infarction ( Fig. 23-1 ). However, the detection of coronary artery calcification is neither sensitive nor specific for ACS.
Exercise Tolerance Testing
A sensitivity of 76% and a specificity of 60% have been described for ETT in the detection of myocardial ischemia. The reported high negative predictive value (98%) of ETT almost completely excludes ACS in the absence of inducible ischemia. One of the advantages of the ETT is its high negative predictive value; however, the test depends heavily on the physical ability of the patient to achieve the necessary heart rate. At least 33% of the patients are not suitable for ETT because of physical restrictions and many other factors.
Echocardiography and Radionuclide Perfusion Imaging
The use of resting echocardiography is limited in that ACS is a dynamic process. Therefore, in the absence of wall motion abnormalities, the examiner cannot exclude myocardial ischemia as a cause of the presenting chest pain episode if the pain is not present at the time of imaging. Conditions such as myocardial stunning can be appreciated on echocardiography because they can result in persistent wall motion abnormalities. Although studies have reported a high negative predictive value of resting echocardiography for the detection of myocardial infarction, conditions such as unstable angina have a considerably lower negative predictive value. In the case of small regions of ischemia or infarction, a segmental wall motion abnormality may not be present.
Because of its prognostic value, stress echocardiography has been well established in the evaluation and triage of patients who present to the ED with acute chest pain. With a reported negative predictive value of 98%, a negative study result in most cases provides a reasonable basis for discharge from the ED and is associated with a very low adverse cardiac event rate. As compared with other techniques, stress echocardiography has many advantages, including portability, the capability to infer myocardial ischemia by demonstrating wall motion abnormalities and reduced ejection fractions, and physiologic information regarding baseline ventricular function, valvular function, and pericardial contour. Nevertheless, stress echocardiography has several disadvantages, including limited availability during off hours, the inability to exclude causes of chest pain outside the heart, limited use in patients with resolved symptoms and nontransmural infarcts, and the potential for a nondiagnostic test result when the necessary heart rate is not achieved. The use of newer technologies such as microbubble echocontrast that have shown to improve endomyocardial border definition and perfusion assessment may ultimately overcome some of the challenges with the use of echocardiography for the assessment of acute chest pain. Thus, stress and rest perfusion imaging has been promoted as an alternative modality.
Findings of multiple studies have shown sensitivity ranging from 90% to 100%, specificity from 60% to 78%, and a negative predictive value from 97% to 100% for radionuclide perfusion imaging for ACS if SPECT imaging is used. The addition of stress imaging in stable patients may improve diagnostic accuracy. In addition, radionuclide perfusion imaging has been shown to have prognostic value and permits risk stratification of patients for future cardiac events.
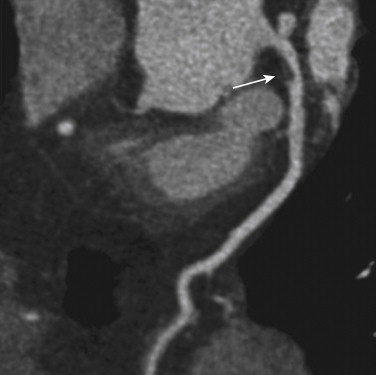
SPECT myocardial perfusion imaging has an important role both in patients at risk for coronary artery disease in the outpatient setting and in those who present with acute chest pain, as demonstrated in Figure 23-2 . SPECT is performed after injection of technetium-99m–based perfusion tracer, with imaging obtained 45 to 60 minutes later, so that stress testing permits assessment of myocardial blood flow at the time of injection. In the ED setting, among low- to intermediate-risk patients with chest pain and nonspecific ECG changes, myocardial perfusion SPECT is effective in excluding ACS. In a series of observational studies, the negative predictive value for ruling out ACS was approximately 99%. Thus, a normal myocardial perfusion study result predicts a very small risk of ACS in this setting.
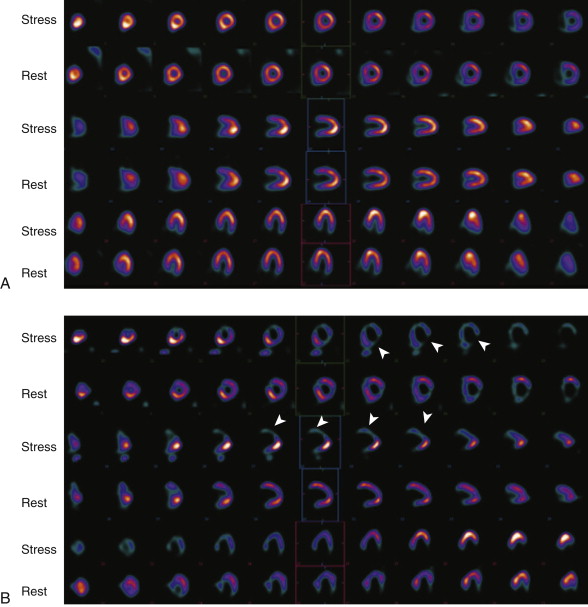
Myocardial perfusion imaging also provides greater risk stratification and prognostic value compared with clinical data for predicting adverse cardiac events. Patients who exhibit abnormal regional perfusion have a higher risk of cardiac events during hospitalization and subsequent to discharge. However, myocardial perfusion imaging has several disadvantages, both practical and intrinsic to the study. Because the test is generally not available during off hours, a patient with potential ACS who presents in the evening must wait until the following morning to be imaged. The nuclear medicine department is often not in the immediate vicinity of the ED suite, thus necessitating removal of the patient to a relatively unmonitored environment. Additionally, myocardial perfusion imaging is prone to artifacts that lead to false-positive results in certain populations. Finally, whereas a negative perfusion imaging study result largely excludes ACS, it does not address the potential for serious noncardiac causes of chest pain such as pulmonary embolism.
Computed Tomographic Calcium Scoring for The Evaluation of Acute Chest Pain
The progression of coronary atherosclerosis is marked by calcification of atherosclerotic plaque. With rapid advances in the technology of MDCT, ECG-gated coronary computed tomography (CT) allows for the detection of the extent and quantification of the calcification, properties that correlate with the presence of obstructive stenosis. In the Agatston method, software highlights pixels with Hounsfield units (HU) greater than 130, and the density and extent of these pixels are combined to create the Agatston score. Although this method was originally developed for the now abandoned electron beam CT, correlation of calcium scores is achieved by using volumetric or mass scoring methods. In symptomatic patients, the sensitivity of the presence of any coronary calcium for a stenosis greater than 50% is 95% to 99%. Calcium detected by coronary CT in symptomatic and asymptomatic patients is predictive of nonfatal myocardial infarction, cardiovascular death, and all-cause mortality. This score is then used to determine the calcium percentile, which compares the calcified plaque burden with that of other asymptomatic men and women of the same age.
The calcium score, in combination with the percentile, enables the physician to determine the patient’s risk of developing symptomatic coronary artery disease and to measure the progression of disease and the effectiveness of treatment. A score of 0 indicates that the patient has no calcified plaque burden. This finding implies an absence of significant coronary artery narrowing and a very low likelihood of a cardiac event over at least the next 3 years, as illustrated in Figures 23-3 and 23-4 . A score of 0 does not absolutely rule out the presence of soft, noncalcified plaque or totally eliminate the possibility of a cardiac event. A score that is greater than 0 indicates at least some coronary artery disease. As the score increases, so does the likelihood of a significant coronary narrowing and the likelihood of a coronary event over the next 3 years, compared with patients with lower scores. Similarly, the likelihood of a coronary event increases with increasing calcium percentiles, as seen in Figure 23-5 .