RV morphology
LV morphology
RV or LV morphology
Hypoplastic left heart syndrome (HLHS)
Tricuspid atresia
Unbalanced AV canal defect
Complex double-outlet right ventricle
Pulmonary atresia
Straddling or crisscross AV valve connections
Double-inlet left ventricle
Heterotaxy
Severe Ebstein’s anomaly
Due to complex anatomy and hemodynamics, any single ventricle patient with Fontan palliation requires continued meticulous medical surveillance. A critical component of this care involves advanced cardiac imaging. A multimodality imaging approach for the Fontan patient is best performed at a center with expertise in adult congenital heart disease and in-depth knowledge regarding the advantages and disadvantages of each imaging technique. Cardiac magnetic resonance (CMR) imaging and multidetector cardiac computed tomography (MDCT) are the noninvasive, advanced cardiac imaging techniques presented here.
The Fontan Procedure
Genesis for single ventricle palliation came with animal studies conducted by Rodbard in 1948, who for the first time successfully bypassed the canine right ventricle by anastomosing the right atrial appendage to the proximally ligated main pulmonary artery [1]. An innovative technique, the cavopulmonary shunt, was first performed by William Glenn in North America on a dog (1954) and shortly thereafter in a patient (1957). It became the standard at several institutions, paving the way for the Fontan palliation for single ventricle patients [2].
The Fontan operation was first described in 1971 as a physiologic corrective surgical procedure for patients with tricuspid atresia [3]. In this original description of the Fontan operation, full oxygenation of blood before returning to the left heart was achieved by direction of systemic venous return to the pulmonary arteries and closing the atrial septal defect (Fig. 21.1a, b).
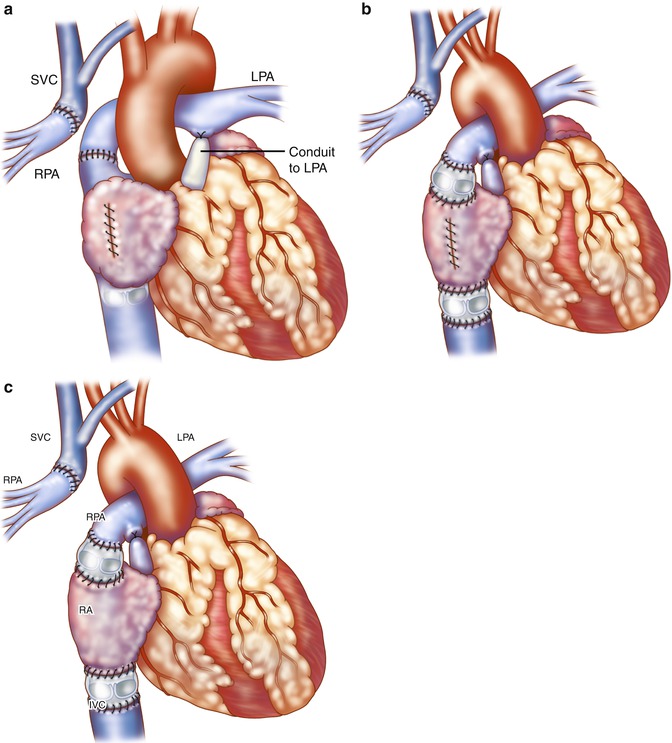
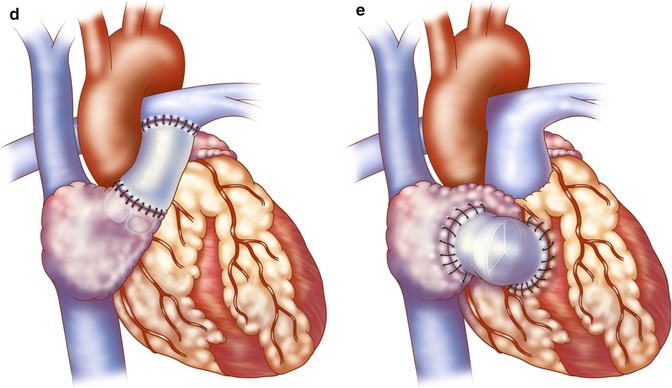


Fig. 21.1
(a, b) Original Fontan operation: the pulmonary artery (PA) is ligated at its connection to the ventricle. (c) The superior vena cava (SVC) is disconnected from the right atrium (RA) and anastomosed to the disconnected right pulmonary artery (RPA) (Glenn procedure). The RA appendage was attached to the left PA (LPA) to direct blood to the left lung directly (a) or via an aortic valve homograft (b). A pulmonary valve homograft is inserted into the inferior vena cava (IVC) and the atrial septal defect is closed (Reproduced with permission from Fontan and Baudet [3]). (d) Kreutzer modification: Anterior connection of the RA appendage to the PA is performed with the patient’s own pulmonary valve or a nonvalved Dacron tube. (e) Björk modification: RA to right ventricle outflow tract anastomosis with a pericardial patch or valved conduit
Since its original description, there have been several modifications to the Fontan procedure, which has been applied to many types of congenital heart disease resulting in single ventricle physiology, regardless of whether cyanosis or pulmonary overcirculation existed initially [4, 5]. In 1973, Kreutzer described maintenance of branch pulmonary artery continuity by direct anterior connection of the right atrial appendage (RAA) to the main pulmonary artery with omission of the Glenn procedure and without a pulmonary valve homograft at the inferior vena cava-right atrium (IVC-RA) junction (Fig. 21.1c). A fenestration was also placed in the atrial septum [6]. Later, Kreutzer performed the posterior atriopulmonary anastomosis, a technique he believed was superior to the anterior anastomosis for reduced patient mortality [7]. The Björk modification incorporated a right atrial to right ventricular anastomosis in tricuspid atresia for potential use of the hypoplastic right ventricle (RV) as a pumping chamber [8] (see Fig. 21.1d). The success of these early procedures has been recognized as one of the major historic advances in surgical treatment for patients with varying types of univentricular heart defects [5, 9]. As these first reports included multiple variations, the term “classic Fontan” is not specific enough, and deviations will be seen in the literature. Thus, it is important to understand the variety of surgical procedures that can be found in adults with single ventricle physiology who have undergone surgical palliation as young children.
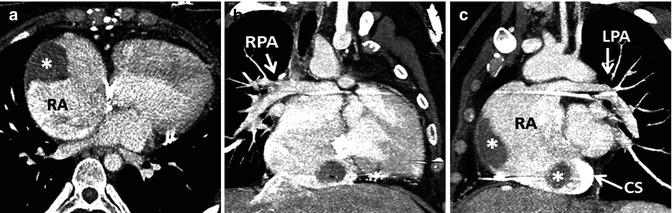
With the Fontan palliation performed in a manner incorporating the anastomosis of the right atrial appendage to the pulmonary artery, long-term complications in many have included significant right atrial dilation along with atrial arrhythmias and/or atrial thrombus formation, often accompanied by ventricular dysfunction and worsening cyanosis, among other issues [10, 11] (Fig. 21.2). In an attempt to address complications encountered in the early Fontan techniques, more recent modifications have included the total cavopulmonary connection reported by de Leval in 1988 [12]. He anastomosed the SVC to the right pulmonary artery (RPA) and created an intra-atrial tunnel formed from the posterior atrial wall and a prosthetic patch, with or without a fenestration, spanning the pathway from the inferior vena cava (IVC) to the SVC-RA junction (Fig. 21.3). As reported in 1990, Marcelletti promoted the extracardiac Fontan, which consisted of a caval conduit entirely outside the atrium from the IVC to the pulmonary artery [13]. The intra-atrial baffle was first described by the Mayo Clinic group and proved useful for patients with anomalous venous drainage [4] (Fig. 21.4). These caval connection modifications have decreased the incidence of complications related to the enlarged right atrium [10, 12, 14].
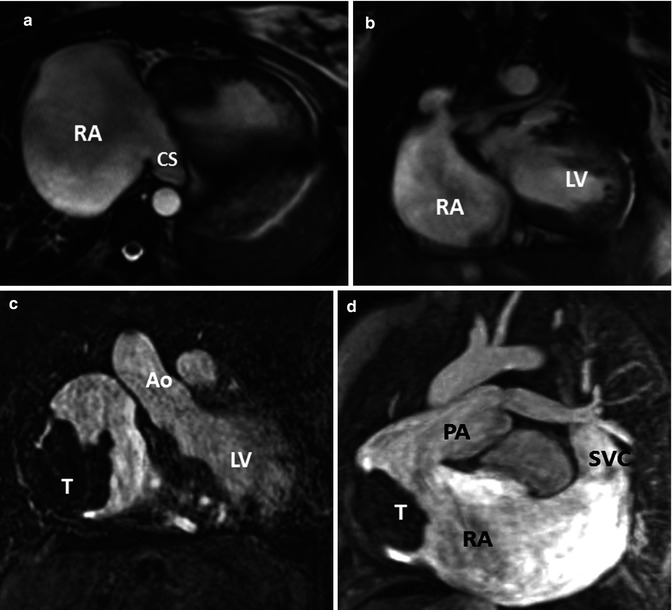
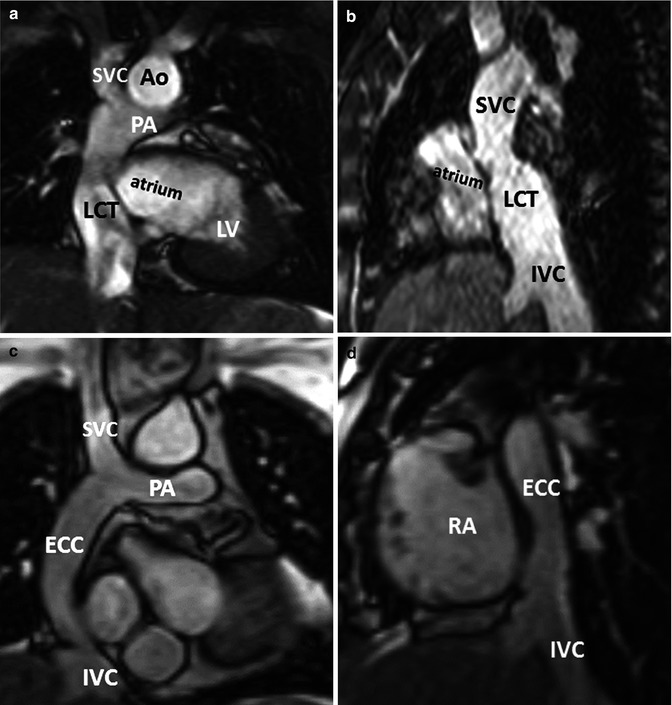
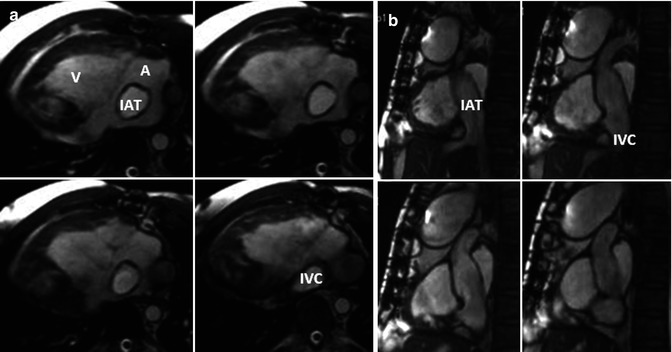

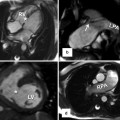
Fig. 21.2
(a, b) A 34-year-old female with tricuspid atresia and history of right Glenn and left Blalock-Taussig shunt as a young child; at age 24 years pulmonary artery (PA) continuity was reestablished along with connection of the right atrium (RA) appendage to the PA. Cardiac MR (CMR) steady-state free precession, bright., Rady Children’s, San Diego, CA. blood images in axial (a) and oblique coronal (b) orientations demonstrate a severely dilated RA and a dilated coronary sinus. (c, d) A 26-year-old male with tricuspid atresia, atriopulmonary Fontan at age 8 years, severely dilated RA, recurrent atrial arrhythmias at age 19 years, treated with amiodarone and warfarin and required electrical cardioversion at age 24 and 25 years, presented for cardiac MR due to fatigue, shortness of breath, and oxygen saturation in the upper 80s and a large RA thrombus (T) was found. Steady-state free precession, bright-blood images in (c) coronal and (d) oblique sagittal orientations. CS coronary sinus, LV left ventricle, Ao aorta, SVC superior vena cava (Courtesy of Beth Printz, M.D)

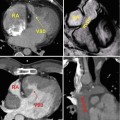
Fig. 21.3
Modified Fontan procedure with total cavopulmonary connections. (a, b) fenestrated lateral caval/intra-atrial tunnel. Oblique coronal (a) and sagittal (b) steady-state free precession, bright-blood CMR images of a 16-year-old female with double-inlet left ventricle (LV) with a fenestrated lateral caval tunnel constructed from prosthetic material and the lateral wall of the right atrium (RA). (c, d) Non-fenestrated extracardiac conduit connection. Oblique coronal (c) and sagittal (d) steady-state free precession, bright-blood MR images of a 27-year-old female with pulmonary atresia, hypoplastic right ventricle, after conversion from atriopulmonary Fontan connection to a non-fenestrated extracardiac Fontan conduit (LCT lateral caval tunnel, PA pulmonary artery, Ao aorta, ECC extracardiac conduit, SVC superior vena cava, IVC inferior vena cava)

Fig. 21.4
Modified Fontan procedure with total cavopulmonary connections. Oblique axial (a) and sagittal (b) steady-state free precession, bright-blood cardiac MR images of a 29-year-old male with dextrocardia, pulmonary atresia, unbalanced atrioventricular canal with bidirectional Glenn, and intra-atrial tunnel. IAT intra-atrial tunnel, IVC inferior vena cava
Completion of the Fontan procedure has now became a two-stage endeavor; first the hemi-Fontan or bidirectional Glenn operation connects the SVC to the branch pulmonary arteries, followed by connection of the IVC via the caval conduit to the branch pulmonary arteries, usually within 2–3 years [15]. In heterotaxy patients with azygous continuation of the IVC to the SVC, the Kawashima procedure [16] is commonly performed to connect the SVC to the pulmonary artery, and later the hepatic veins are joined to the pulmonary circulation, preventing the development of pulmonary arteriovenous malformations [17, 18] (Fig. 21.5).
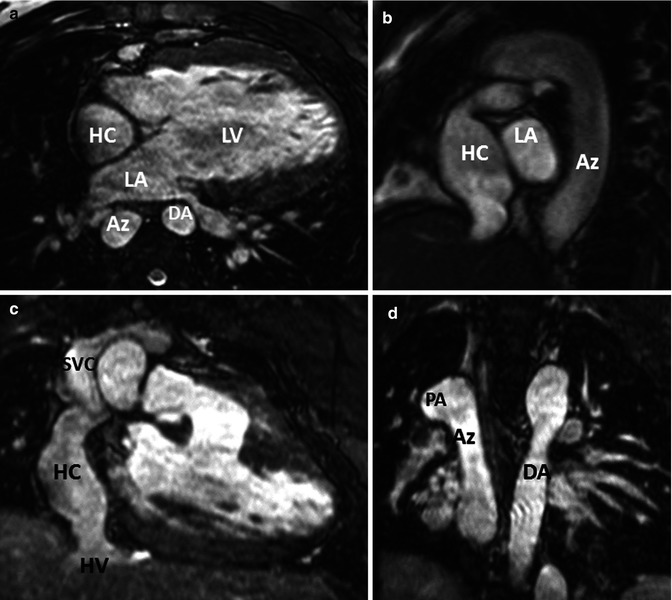

Fig. 21.5
Images from a 3D steady-state free precession, bright-blood acquisition using ECG gating and respiratory (diaphragm) triggering. A 19-year-old male with double-inlet left ventricle (LV), l-transposition, azygous (Az) continuation of inferior vena cava (IVC), aortic coarctation after coarctation repair at age 8 days, Kawashima procedure at age 20 months, and completion of Fontan procedure at age 3 years with intra-atrial patch tunnel of the hepatic veins (HV) to right pulmonary artery (PA). (a) Axial, (b) oblique sagittal, (c) anterior coronal, and (d) posterior coronal orientations demonstrating hepatic conduit (HC) to the right PA, azygous continuation of the IVC and the descending aorta. LA left atrium
While over the years, there have been improved clinical outcomes immediately postoperative and improved patient prognosis and survival into adulthood [12, 19, 20], complications and hemodynamic problems continue to be seen in the Fontan patient with single ventricle physiology [21, 22]. These include pleural effusions, thromboembolism, atrial dilation and dysrhythmias, conduit stenosis and dilation, exercise intolerance, ventricular failure, pulmonary arteriovenous malformations, aortopulmonary collaterals, venovenous collaterals, protein-losing enteropathy, and plastic bronchitis [11, 14]. Many older patients have experienced relief of symptoms with conversion of their original Fontan atriopulmonary connection to a lateral atrial tunnel or extracardiac conduit, often in conjunction with atrial reduction and/or a Maze procedure to address atrial arrhythmias [10].
Approach to MDCT for the Patient with Fontan Palliation
It is generally preferable to use echocardiography and/or CMR as a first approach to obtain adequate morphologic and functional information when imaging Fontan patients. As echocardiography becomes more difficult in older and larger patients who have had multiple chest surgeries, CMR assumes an important role in noninvasive evaluation of Fontan patients for serial follow-up to assess patency of the systemic venous to pulmonary artery pathways, ventricular function, and flow quantification. MDCT is most often utilized if evaluation of the postoperative vascular morphology is limited on MRI because of susceptibility artifacts from indwelling ferromagnetic materials such as stents, coils, and occlusion devices. Major artifact from vascular occlusion coils obscuring the heart and vasculature can be seen in up to 36 % of Fontan patients [23] (Fig. 21.6). MDCT can be used as an alternative imaging method in patients with indwelling pacemakers and retained pacing wires, which remain as contraindications for CMR. MDCT angiography can provide accurate imaging of the extracardiac vasculature that is comparable to MRI with shorter procedure times and less need for sedation and general anesthesia [24]. MDCT acquisitions with ECG gating or triggering can assess intracardiac and coronary morphology and ventricular function. A significant limitation of using MDCT to evaluate Fontan patients is its current inability to provide flow information to quantify valvular regurgitation, pulmonary and systemic blood flow, and aortopulmonary collaterals. Therefore, it is preferable to use CMR as opposed to MDCT if there are no contraindications or artifacts on CMR.
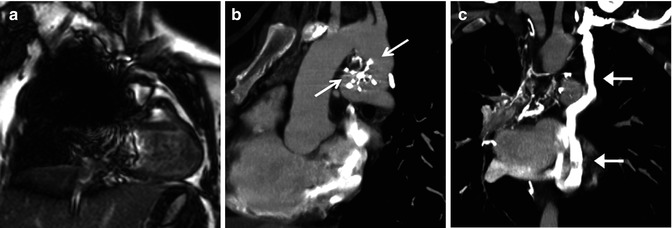

Fig. 21.6
Fontan patient presenting with cyanosis. This patient has a history of prior occlusion of a PDA with a Clamshell device (arrows in b) which resulted in a large artifact obscuring most of the mediastinum on MRI (a) but caused very little artifact on subsequent CT (b) sagittal oblique image). A coronal reformatted CT (c) reveals the source of the cyanosis to be a large systemic to pulmonary venous collateral (arrows) from the left innominate vein to the left-sided pulmonary veins
Technical Considerations
Cardiovascular imaging using current MDCT technology applies fast tube rotation times to decrease cardiovascular motion artifacts and routinely produces images with sub-mm isotropic spatial resolution. Increased emphasis on radiation dose reduction with improved scanner design over the past 10 years has significantly reduced patient doses with MDCT imaging [25]. Of the many factors that can affect radiation dose, the most important for reducing radiation exposure are to use techniques that adjust the tube current according to the weight of the patient [26], automatic tube current modulation to automatically adjust the tube current based on the size, shape and density of the body part being scanned [27], and fast gantry rotation times [28, 29]. In the past few years, iterative reconstruction of CT images has made a substantial impact on lowering patient doses, in addition to improving image quality by reducing image noise due to streak artifacts from calcification and metallic artifacts [30–33].
Higher patient doses are generally associated with ECG-gated acquisitions which are not needed for routine angiography of the thoracic vasculature. ECG gating is necessary for evaluation of the aortic root and ascending aortic dimensions, coronary arteries, and ventricular function, and therefore, cardiac MDCT angiography with ECG-gated acquisitions should be restricted to these applications. Retrospective ECG gating is used to obtain images throughout the entire cardiac cycle for quantification of ventricular function, wall motion, and motion of valves [34], and the measurement of ventricular volumes, mass, and function has shown good correlation with measurements from CMR [35]. Retrospective ECG gating delivers a relatively high radiation dose because the x-ray tube is on throughout the cardiac cycle to acquire imaging data, and reconstructing multiple phases requires oversampling of image data and results in longer scan times. When imaging the aorta and coronary arteries, radiation doses can be substantially reduced by using prospective ECG triggering instead of retrospective ECG gating. With prospective ECG triggering, scanning is initiated at a predefined moment in the cardiac cycle from the QRS complex, usually in diastole. This technique delivers a low radiation dose because the x-ray tube is only turned on during a predefined portion of the cardiac cycle [36]. MDCT scanners with 320 detector rows (volumetric scanners) or second-generation dual-source scanners have become available with faster scanning so that sedation and breath holding are no longer necessary [37], and gated acquisitions for assessment of the heart and coronary arteries have significantly improved temporal resolution and allowed substantial reduction in radiation doses [38–40].
An important consideration when performing MDCT angiography of Fontan patients is the timing of the contrast injection and initiation of scanning [41]. Because the flow through the Fontan circulation is passive from the systemic venous system to the pulmonary arteries, homogeneous enhancement of the Fontan pathway cannot be obtained until the venous phase of contrast administration is reached. A common error is to commence scanning a Fontan patient with a standard protocol for routine pulmonary MDCT angiography when the pulmonary arteries just begin to be opacified. Scanning this early will result in inhomogeneous enhancement of the Fontan pathway (Fig. 21.7). There are several options for timing the contrast administration and scanning depending on the information that is needed from the examination and the intravenous access that can be established. Imaging to assess the Fontan pathway and pulmonary artery patency and possible thrombus requires homogeneous opacification which can be obtained with administering contrast via an upper or lower extremity IV and scanning at 50–70 s. Bolus tracking of the IVC for an upper extremity injection and bolus tracking of the SVC for a lower extremity injection can be used, but care must be taken to delay the initiation of the bolus tracking during the contrast injection in order to keep the radiation dose low. Based on this author’s experience, a 60-s scan delay routinely provides adequate contrast opacification of all of the intrathoracic vasculature with only minor inhomogeneity (Fig. 21.8). Others have found that waiting until 3 min after contrast administration provides the most homogeneous contrast opacification [42], but this is at the expense of overall reduction of contrast density, which can make image interpretation difficult, especially if low radiation dose protocols are utilized. Another option is to administer contrast with simultaneous injections of the upper and lower extremity, which allows denser opacification of the entire Fontan circulation but can be difficult if there is poor IV access. For detection of aortopulmonary collaterals, which can be a source of life-threatening hemoptysis, an earlier arterial phase scan for dense opacification of the aorta is required (Fig. 21.9).
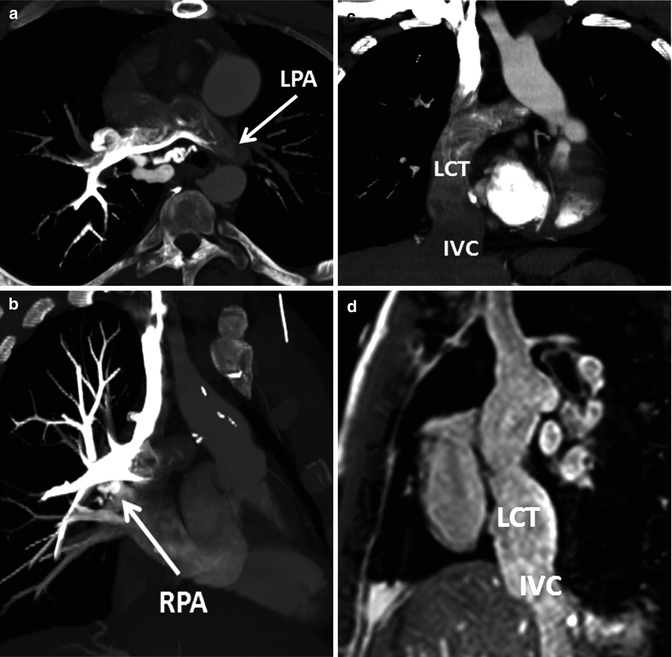

Fig. 21.7
Example of inhomogeneous opacification of the Fontan circulation on CT. Patient with congenitally corrected transposition of the great arteries (TGA) and straddling tricuspid valve status post lateral tunnel Fontan presented with chest pain and concern for pulmonary embolism. Scanning was initiated during bolus tracking when the contrast had just started to enhance the pulmonary arteries, resulting in inhomogeneous opacification of the central pulmonary arteries (oblique axial image a) and preferential enhancement of the right pulmonary artery (RPA) from an upper extremity injection (oblique coronal image b). The lateral conduit tunnel (LCT) from the inferior vena cava (IVC) to the RPA (coronal image, c) has unopacified blood from the IVC simulating extensive filling defect from thrombus. (d) An oblique sagittal steady-state free precession image from a subsequent MRI shows a patent LCT with no intraluminal thrombus. LPA left pulmonary artery