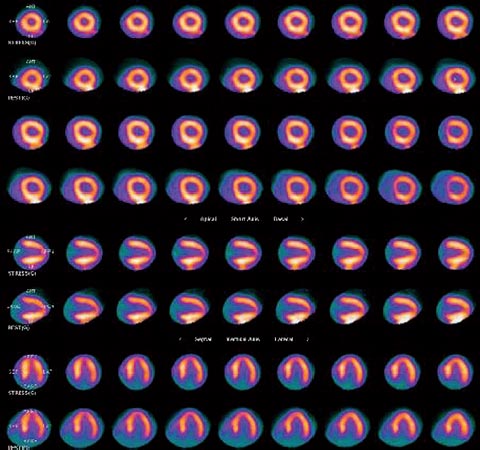
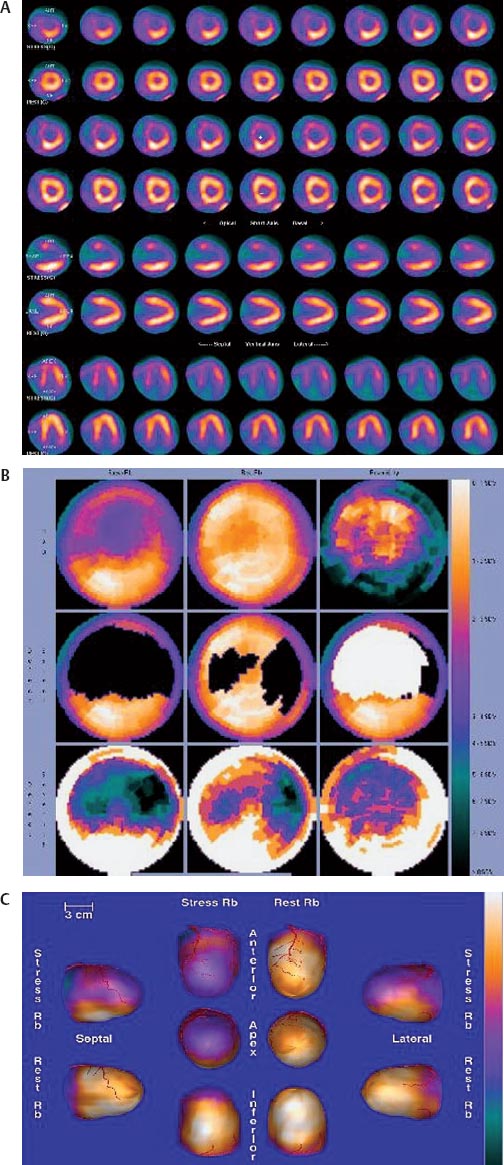
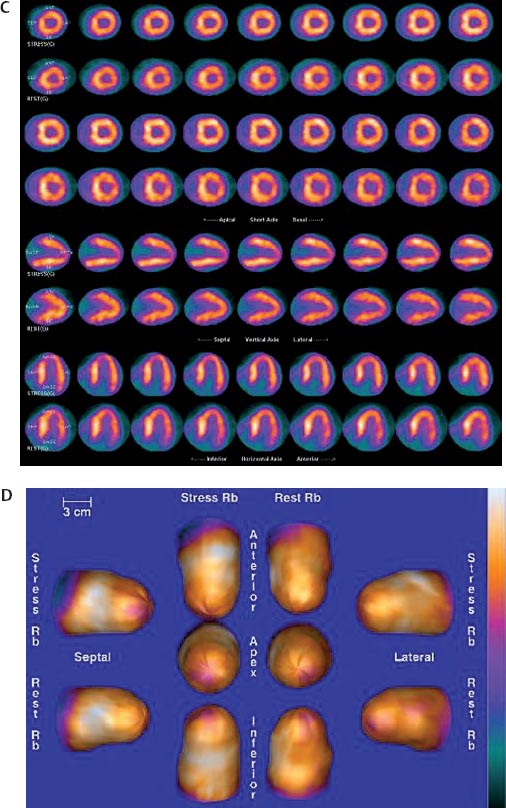
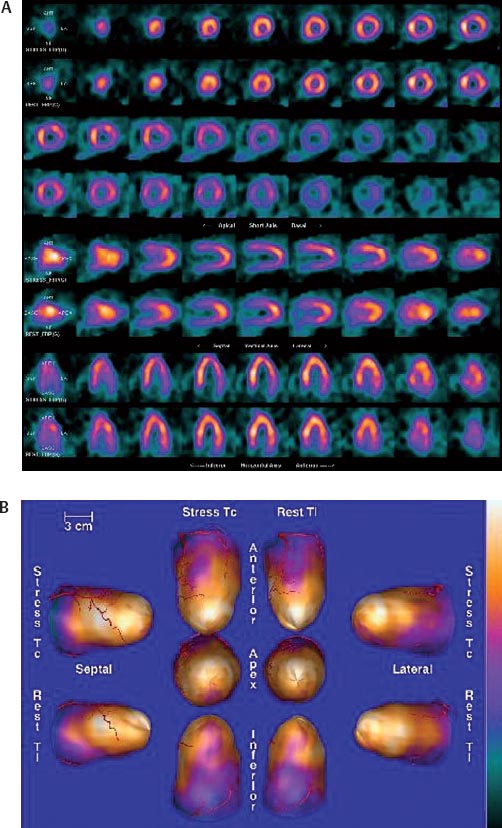
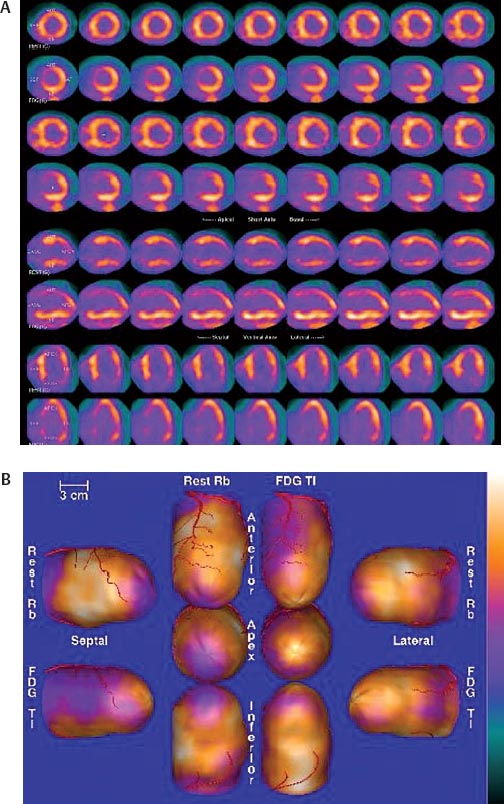
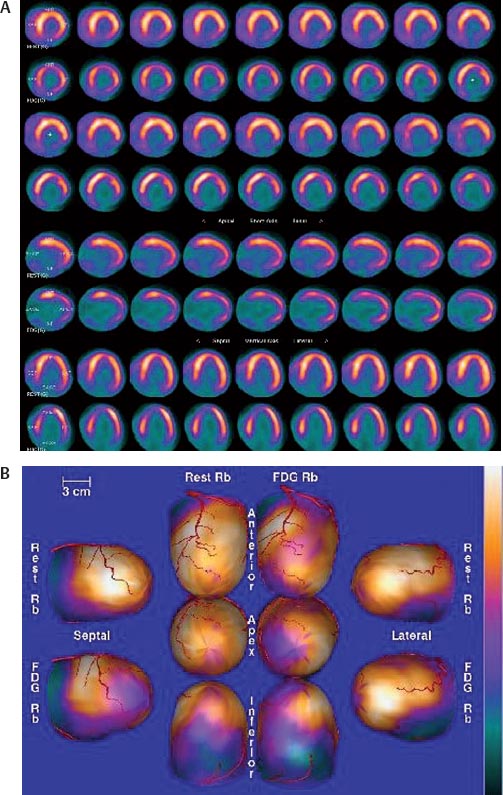
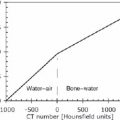
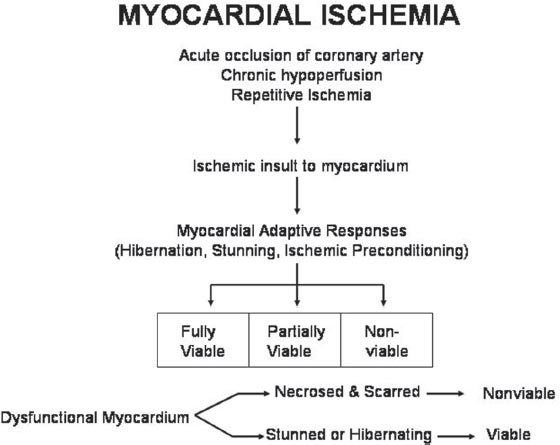
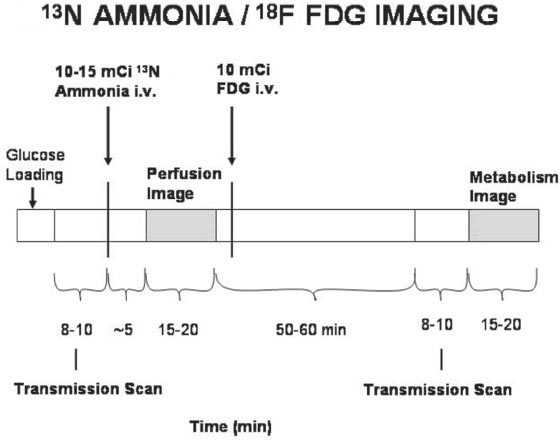
30 Myocardial perfusion assessment at rest and with stress (exercise or pharmacologic) is important in patients with known or suspected coronary artery disease (CAD). Single-photon emission tomography (SPECT) myocardial perfusion imaging with thallium or technetium 99m labeled agents is routinely practiced. However, SPECT imaging has several limitations and myocardial perfusion imaging with positron emission tomography (PET) is superior to SPECT. In practice, PET myocardial perfusion imaging is constrained by the need for an on-site cyclotron to perform nitrogen 13 ammonia imaging. Rubidium 82 imaging can be performed with a generator. Given the cost of the generator and current reimbursements, 2 to 3 studies a day may need to be performed for PET to be cost-effective. Nitrogen 13 (13N) ammonia (cyclotron-produced) and rubidium 82 (82Rb) chloride (generator produced) are Food and Drug Administration (FDA)-approved PET radiopharmaceuticals for assessing myocardial blood flow. Oxygen 15 (15O) labeled water and 62Cu-pyruvaldehyde bis (N4-methylthiosemicarbazone) (Cu-PTSM) can also be used but are mostly restricted to the research setting. 82Rb can be eluted from a strontium 82 generator, which needs to be replaced approximately every 4 weeks. The half-life of 82Rb is 75 seconds. 13N ammonia has a half-life of ~10 minutes. The advantages of 82Rb are that a cyclotron is not needed, and it is ideal for peak dipyridamole or regadenosan stress gated imaging. Rb-82 as imaging typically starts 90 to 120 seconds after injection compared with 3 to 5 minutes with 13N ammonia. The disadvantages are poorer resolution (due to a positron range of 2.6 mm), lower extraction, and more difficulty with quantitation compared with 13N ammonia. It is also very difficult to perform exercise stress testing with 82Rb, given the short half-life of the tracer. Generally, similar protocols (except for dose of radiotracer and imaging time and duration) to those used for SPECT are followed; however, usually pharmacologic stress testing is performed. Exercise stress testing is difficult due to the short half-life of the tracers. With 13N ammonia, exercise stress testing can be done, but it requires meticulous coordination and setup. The images are displayed and reviewed similar to the cardiac SPECT images (Fig. 30.1 and Fig. 30.2). PET imaging provides better spatial and temporal resolution and hence is better suited in patients with thick/muscular chest wall, large breast tissue, or overall body habitus that frequently leads to indeterminate SPECT myocardial perfusion studies (Fig. 30.3). Due to the short half-life of the PET tracers, pure stress-related images uncontaminated by the prior rest injection can be obtained. Unlike gated SPECT, left ventricular ejection fraction can be assessed at peak stress, as imaging is performed soon after injection. PET imaging, with absolute quantification of regional radiotracer uptake, is better suited when serial studies are required to assess perfusion in a particular myocardial segment. It also allows better evaluation of endothelial dysfunction and coronary flow reserve as a measure of coronary stenosis. Moreover, PET allows for more efficient imaging protocols, leading to faster studies (around 45 minutes compared with 3 to 4 hours for SPECT studies) and lower radiation exposure. Numerous studies have shown that myocardial perfusion PET has higher accuracy,2 sensi-tivity,3 and specificity4 compared with SPECT. Patients with severe LV dysfunction and CAD continue to pose a significant management dilemma to clinicians who frequently need to choose between aggressive medical treatment and revascularization therapy.5 Revascu-larization therapy results in better long-term survival rates, and several investigators have demonstrated the benefit of revascularization in CAD patients with poor LV function.6–14 However, it is associated with significant periprocedure morbidity and mortality, making identification and selection of only those patients who will benefit maximally from revascularization extremely crucial. Improvement in the LV function after revascularization is mainly dependent on the reversibility of contractile dysfunction. Dysfunctional but “viable” myocardium is said to be reversibly dysfunctional, whereas scar tissue usually results in nonreversibly dysfunctional myocardium. Thus, accurate identification of myocardial viability is a critical component in the diagnostic work-up of these patients. Myocardial ischemia may result from acute coronary artery occlusion or from chronic hypoperfusion or repetitive ischemic processes. The severity and duration of myocardial ischemia will determine the myocardial response to the ischemic process. Although the myocardium has several immediate as well as sustained mechanisms of adaptations (e.g., hibernation, stunning and ischemic preconditioning15–17) to withstand acute and chronic ischemia, the end result of ischemic injury is a mechanically dysfunctional myocardium. The dysfunctional myocardium may be related to ischemic but viable myocardium, such as stunned or hibernating myocardium or necrosed and scarred myocardium that may be completely nonviable (Fig. 30.4). Fig. 30.4 Myocardial response to ischemia. Fig. 30.5 Protocol to assess myocardial viability with positron emission tomography. FDG, fluorodeoxyglucose. The normal myocardium preferentially uses free fatty acids (FFAs) as energy substrates during normal fasting conditions. During hypoxia and ischemia, FFA oxidation is markedly decreased, and the rate of anaerobic glycolysis is enhanced. Thus, the ischemic myocardium uses glucose in preference to FFAs as the energy substrate.18–27 FDG PET can reliably and accurately assess the initial steps of glucose metabolism in the ischemic myocardium by evaluating myocardial glucose uptake. The protocol for assessing myocardial viability using PET and 13N ammonia for myocardial perfusion imaging and FDG for myocardial metabolism imaging is depicted in Fig. 30.5.
Cardiac PET and PET/CT
Amol Takalkar, Eugene C. Lin, Elias Botvinick, Adam M. Alessio, and Luis Araujo
 Myocardial Perfusion Assessment
Myocardial Perfusion Assessment
Clinical Indication: A
Tracers
Protocols
Accuracy and Comparison with Other Modalities
Pitfalls
 Myocardial Viability
Myocardial Viability
Clinical Indication: A
Clinical Scenario
Mechanism of Myocardial Ischemia
Identification of Myocardial Viability by PET
| Imaging Method | Sensitivity % | Specificity % |
| PET | 93 | 58 |
| Thallium | 86 | 59 |
| rest-redistribution | ||
| Thallium reinjection | 88 | 50 |
| Technetium tracers | 81 | 66 |
| Dobutamine echo | 81 | 80 |
Abbreviations: echo, echocardiogram; PET, positron emission tomography.