History
Since their inception in the 1950s, cardiac valve prostheses have undergone remarkable improvements, yet their fundamental construction has remained largely unchanged. The prosthetic cardiac valve is inextricably linked to the development of extracorporeal circulation, which was also being designed during the same period, in the early 1950s. The use of extracorporeal circulation permitted the delivery of cardioplegia to arrest cardiac motion temporarily for placement of valvular prostheses, among other indications.
The first artificial valve was placed by Dr. Charles Hufnagel in the descending thoracic aorta for the treatment of a patient with aortic insufficiency. Although this effort was only minimally successful, rapid advances in the field led to the first successful artificial valve implantation, which was performed in 1960 by Dr. Dwight Harken and involved placing a caged ball valve in a subcoronary position. Since that initial surgical procedure, cardiac valves have been placed in situ in hundreds of thousands of patients worldwide. A concomitant decrease in mortality has also occurred, from almost 20% in the early years of cardiac valve implantation to less than 2% in most centers worldwide today.
More than 70 different types of both mechanical and bioprosthetic valves have been placed since the inception of valve replacement surgery. In practice, however, only a few valves are commonly used at present, and these include the Starr-Edwards ball valve, the Omniscience, Omnicarbon, and Medtronic Hall tilting disk valves, and the St. Jude and Carbomedics bileaflet valves.
Imaging
Imaging of cardiac valve prostheses has been an integral part of evaluation since the mechanical valve itself was first introduced. Knowledge of the different valve prostheses allows the cardiac imager to make an accurate determination about the function and placement of the valve prosthesis, either mechanical or bioprosthetic.
Initial evaluation of valve prostheses was limited to the use of radiographs and was largely confined to anatomic delineation. With the advent of more advanced echocardiographic techniques, as well as improvements in computed tomography (CT) imaging and magnetic resonance imaging (MRI), however, noninvasive prosthetic valvular assessment became possible. More invasive imaging in terms of both anatomic delineation and function is also possible with cardiac catheterization.
Caged Ball Valves
The caged ball valve was the original mechanical heart valve. Over the years, several iterations have provided variations on the theme of a ball in a cage. Various attempts have been made in each of these designs to develop better cage mechanisms by using different alloys such as titanium and by altering the configuration of the cage itself, such as incorporating double cages. The ball within the cage was also changed several times to incorporate different alloys and different combinations of nylon and silicone in attempts to reduce ball variance. A significant improvement in ball technology occurred when the silicone ball was heat cured, a process that essentially eliminated variance, which was one of the main problems of the earlier designs.
Hufnagel Aortic Ball Valve
The first valvular prosthesis placed by Dr. Hufnagel was a methacrylate ball contained in a methacrylate tube. The ball sat in the proximal portion of the tube in diastole, and three outpouchings opened during systole and allowed for unidirectional flow of blood across the valve. The valve was placed in the descending aorta and was designed for use in patients with aortic insufficiency.
More than 200 individuals received the Hufnagel prosthesis. No anticoagulation was used. Hufnagel ball valves recovered 30 years after implantation showed no wear. The prosthesis remained essentially untouched from its original design, except for the replacement of the methacrylate ball with a hollow nylon ball that was coated in silicone to reduce valve noise.
Harken-Soroff Ball Valve
Originally introduced in 1960, the Harken-Soroff ball valve consisted of a Magovern turtleneck sewing ring covered by a Dacron skirt to facilitate easy insertion. Approximately 2300 patients had Harken valves inserted in the late 1960s and early 1970s. The operative mortality and long-term risk of thrombosis were both reported to be approximately 7%. The valve itself was found to suffer from variance and disk wear. At the time of its inception, it had a significantly lower rate of complication than the Starr-Edwards valve.
Bahnson Fabric Cusp Valve
Originally introduced in 1960, the Bahnson valve consisted of Teflon knit aortic cusps. Anywhere from one to three cusps could be replaced with this design. Although the initial evaluation of the efficacy of the valve was successful, ultimately the valve was limited by stiffening and tearing of the leaflets, which often occurred within the first 24 months because of fibrin deposition and ingrowth of connective tissue.
Magovern-Cromie Ball Valve
Originally introduced in 1962, the Magovern-Cromie ball valve (Pemco, Cleveland, Ohio) consisted of a caged ball valve apparatus that offered the distinct advantage of sutureless fixation with a pin system. The valve was in production from the early 1960s to 1980 and is no longer routinely used. The valve offered the benefit of allowing replacement with relatively short cardiopulmonary bypass times, an advantage at the time when cardiopulmonary bypass technology was at an early stage. In a 13-year follow-up study, the main identified risk was thromboembolic disease. A report from Magovern in 1989 delineated his experience with the procedure and found an 11% rate of isolated aortic valve replacement (AVR) and a relatively low risk of postoperative complications. In his 25-year review, Magovern was able to conclude that the valve was both safe and durable.
Smelloff-Cutter (SCDK) Ball Valve
Originally introduced in 1964, the Smelloff-Cutter ball valve incorporated a double cage design to hold the ball more in the equator of the apparatus. Long-term efficacy of this valve also proved to be quite good. At 25 years, the rates of valvular dysfunction, reoperation, and endocarditis were reported to be 1.16%, 1.16%, and 0.2%, respectively, per patient-year. Furthermore, no surviving patient deteriorated over the reported time in his or her functional New York Heart Association classification. Current reports have noted intact Smelloff-Cutter valves up to 37 years after implantation that are in good condition. The longest reported valve duration was 43 years; however, at that point the valve had to be retired because of lipid infiltration and pannus formation.
DeBakey-Surgitool Valve
The DeBakey-Surgitool valve was introduced in 1967. It was on the market from 1969 to 1978, and approximately 3300 valves were implanted worldwide. The valve represented progress in technology given that it was the first valve to introduce pyrolytic carbon, which provided superior durability and biocompatibility. Ultimately, the valve was discontinued because of its propensity for strut fracture; however, case reports have noted valve durability for more than 30 years.
Braunwald-Cutter Valve
The Braunwald-Cutter caged ball apparatus was first introduced in 1960. The original design incorporated flexible Teflon-coated chordae tendineae. Unfortunately, in the initial offering, the mitral and aortic fabric prostheses became stiff and immobile. Recovered specimens demonstrated covering and infiltration with a tightly adherent layer of fibrous connective tissue. The model was then revised and was reissued in 1968 with struts covered with a knit Dacron tubing and an inflow ring covered with ultrathin polypropylene mesh fabric. This second model was found to suffer from significant cloth and poppet wear, particularly in the aortic position. In several instances, the poppet escaped from the apparatus and embolized. Mitral position valves had better outcomes, likely because of the lower pressures in this position.
Starr-Edwards Valve
The Starr-Edwards valve (Edwards Lifesciences, Irvine, Calif.) was introduced in 1960. The design was much the same as that of other caged ball valves. Between the initial introduction of this valve in 1960 and 1982, several modifications were made to achieve greater stability. These included changing the original Lucite cage to a metal cage and changing the ball from silicone to a hollow satellite ball. Eventually, this ball was replaced with a heat-cured ball that all but eliminated the problem of variance ( Figs. 17-1 and 17-2 ). The fundamental construction of this valve has remained essentially unchanged for more than 30 years.
In intermediate-term follow-up at approximately 8 years, 95% of patients had only New York Heart Association class I or class II symptoms. Same study had no reports of valve thrombosis or late valve-related deaths. In a larger study, Orszulak et al reported on 1100 patients who underwent valve replacement with the 1260 Starr-Edwards valve. This study demonstrated the absence of thromboemboli and of anticoagulant-related bleeding at 5 years to be 90.8% and 98.7%, respectively. Survival from all-cause mortality in this patient population was 76.6%, 59.6%, 44.9%, and 31.2%, at 5, 10, 15, and 20 years, respectively, including operative mortality in this study with median age of 57 years at the time of implantation.
Case reports of the Starr-Edwards valve more than 30 and even 40 years after implantation show excellent durability and no structural valve degeneration. Furthermore, these valves are generally free of thromboembolic or bleeding complications related to anticoagulation.
The initial imaging strategy in the evaluation of the Starr-Edwards valve is typically echocardiography. Comprehensive data on Doppler echocardiographic assessment of the function of the normal Starr-Edwards mitral valve prosthesis are available. Detection of complications related to Starr-Edwards valves is also the typical starting point for evaluation, with reasonable detection rates. Echocardiography has been used to detect cloth tears, thrombosis, and paravalvular regurgitation. Transesophageal echocardiography is typically more sensitive for the detection of complications than is transthoracic echocardiography. Cardiac CT and MRI are potential problem-solving tools to delineate complications seen on echocardiography more clearly or to look for complications in patients when reasonable clinical suspicion exists. Reports of detection of complications related specifically to the Starr-Edwards valve have not been published. The reader is referred to the later section on complication detection for a discussion of the current literature on this subject.
Tilting Disk Valves
The 1960s saw the development of tilting disk valves. These valves have also undergone several design changes since the first clinically available valve in 1969. The typical design of a tilting disk valve is a circular disk that opens and closes and is controlled by a metal strut. Typically, the valve consists of a metal ring covered by fabric. The disk is made of pyrolytic carbon because of the superior longevity and biocompatibility of this material. The main advantage of the tilting disk design is the ability to allow restoration of central blood flow, as opposed to the ball in cage design.
Björk-Shiley Valve
The Björk-Shiley valve (Shiley, Irvine, Calif.) was the first of the low-profile tilting disk valves to be introduced. Two types of valves were marketed. A flat disk model ( Fig. 17-3 ) was introduced in 1969, and a convexoconcave design was subsequently marketed in 1975. In total, approximately 300,000 flat disk valves were inserted in both the mitral and aortic positions, and approximately 86,000 convexoconcave valves were implanted in the same positions. The convexoconcave design was developed to improve flow across the valve, but it led to strut fracture in approximately 2% of cases. This complication led to a class action law suit and also necessitated prophylactic replacement in several patients.
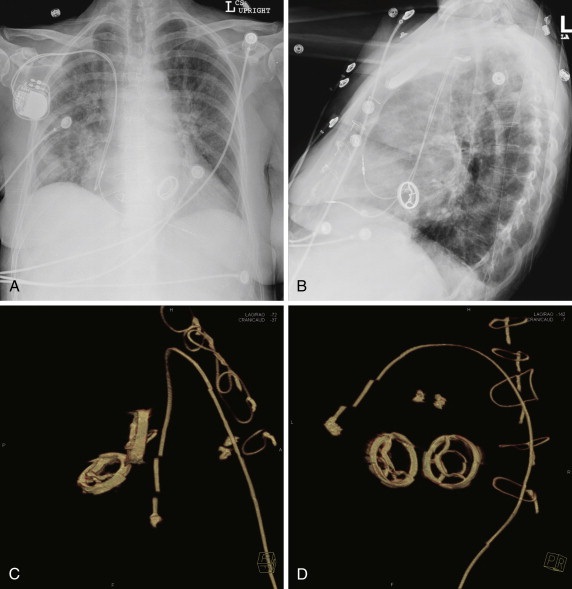
In the early valves, the disk material was made of Delrin, which is an acetal resin. Initially, this compound was thought to provide stability for more than 50 years of use. However, over time, the disk became susceptible to distortion from steam absorption. Subsequent cases of valve deformity and dysfunction were reported. The valve was revised to change the disk to pyrolitic carbon in 1971, but the initial version was sold until 1979. The popular flat pyrolytic disk model was found to suffer from an increased risk of thrombosis, and therefore the convexoconcave model was subsequently introduced (see Fig. 17-3 ).
The Björk-Shiley convexoconcave disk was constrained within the device by inlet and outlet struts. Although the inlet strut was integral to the device, the outlet strut was welded to the apparatus. The purported mechanism was excessive force during closing of the disk that was almost 10 times the force experienced during disk opening. The stresses on the outlet strut were greater than the strut wire’s fatigue endurance limit. The resulting complications proved catastrophic. Fractures of the valve’s outlet struts resulted in escape of the disk, which led to consequent embolization, massive regurgitation, and often death. Sixty- and 70-degree models were introduced. The 70-degree model was withdrawn from the market in 1983, and the 60-degree model was withdrawn in 1986. The withdrawal of these devices from the market posed a significant risk to the roughly 86,000 patients who had already undergone valve replacement, and therefore reoperation was considered in a subset of patients.
The epidemiologic data identified four factors that contributed to valve failure: opening angle, valve size, mitral position, and young age. Several iterations of decision support about which patients should receive prophylactic valve replacement have been made. A second study concluded in 1998 allowed updating of the guidelines. A 25-year follow-up study on findings after valve replacement is now available. Based on the study by Blot et al, it appears that, in most patients, the risks of reoperation far outweigh the benefits. However, outlet strut fractures do continue to occur even 25 years later. Guidelines have been developed to identify the small percentage of patients who would be expected to have a gain in life expectancy should reoperative surgery be performed. These patients are mainly young men. The risks of outlet strut fracture tend to decrease with increasing age.
Some research on imaging of strut fracture related to these valves has been conducted. O’Neill et al demonstrated that strut fractures can be visualized using high-resolution cineradiography; however, this technique was unable to visualize all strut fractures. This finding led the authors of that study to conclude that although the risk of strut fracture is not entirely eliminated by cineradiographic screening, it can be reduced. In practice, the decision to reoperate is guided mainly by epidemiologic study. No reports of CT or MRI screening to identify those patients at risk of strut fracture have been published.
Medtronic Hall Valve
Originally introduced in 1977, the Medtronic Hall (Medtronic, Inc., Minneapolis, Minn) valve represented an improvement on the tilting disk valves of the day. The design incorporates a Pyrolite disk with a small central hole or perforation. The disk perforation slides over a guidewire, to tilt to the open position ( Figs. 17-4 and 17-5 ). The housing for the disk is made of titanium with a Teflon sewing ring. In contrast to the Björk-Shiley valve, the Medtronic Hall valve contains no welds. In the aortic position, the valve opens to 75 degrees. To date, more than 300,000 of these valves have been placed, and the valve is still in production today. The valve roots originated in Norway, and is the most commonly placed tilting disk valve in the United States.

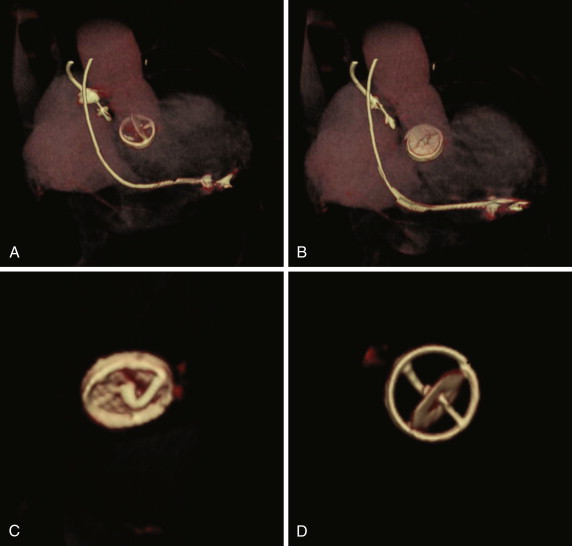
Long-term follow-up is available for up to 25 years. In their report of a 25-year experience Svennevig et al found no report of mechanical failure of the valve in their cohort of 816 patients. Furthermore, the rates of complication were 1.5% per patient-year for thromboembolic complications, 0.7% per patient-year for warfarin-related bleeding, and 0.16% per patient-year for endocarditis. In another study, Butchart et al found results similar to those of the study by Svennevig et al, with linearized rates of valve-related late death for AVR, mitral valve replacement (MVR), and double valve replacement of 0.8%, 0.9%, and 1.1% annually, respectively. The rates of adverse events were up to 0.04% per year for valve thrombosis, up to 4.0% for all cases of thromboembolism, up to 0.8% per year for stroke, up to 1.6% for major hemorrhage, and up to 0.7% for prosthetic endocarditis, depending on the position of valve replacement. In contrast, in a smaller study of ethnic Koreans, Cho et al found higher rates of thrombus and pannus formation than reported in the foregoing studies; these complications were attributed to smaller valve sizes implanted in their patients compared with the others.
The goal of the Medtronic Hall design was to improve on previous tilting disk designs, with the aim of improving durability and hemodynamic performance and reducing thrombogenicity. The results of these long-term studies show that these aims have largely been achieved.
The Medtronic Hall valve has also proven to possess superior hemodynamic characteristics, which are intrinsic measures of valve performance. Echocardiographic assessment showed that the hemodynamic performance of the Medtronic Hall valve was significantly better than that of Björk-Shiley and Starr-Edwards prostheses of the same size. Moreover, in 29- or 31-mm prostheses, the hemodynamic parameters were similar to those of native mitral valves. Furthermore, Buthcart et al found that in those patients whose dominant lesion was aortic stenosis without coronary disease, survival was almost identical to that of the age- and sex-matched general population for the first 7.5 years.
The flow characteristics of the Medtronic Hall valve offer relief of transvalvular gradients and therefore allow left ventricular (LV) mass regression after AVR. Furthermore, optimal blood flow across the valve is also thought to reduce the risk of thrombus deposition and platelet activation, thus accounting for the relatively low rates of valve thrombosis. The Medtronic Hall valve has been shown to have superior hemodynamic performance compared with the St. Jude or Monostrut mechanical valves in patients with valves smaller than 20 mm. When these valves are optimally placed, less turbulent flow in comparison with the bileaflet design may contribute to the trend toward greater LV mass regression that has been reported with the Medtronic Hall valve.
CT studies of the Medtronic Hall valve itself are limited. However, in a previous study, investigators showed, using electron beam CT analysis, that Medtronic Hall valve implantation allowed for a reduction in LV mass in patients with small aortic roots.
Lillehei-Kaster, Omniscience, and Omnicarbon Valves
The Lillehei-Kaster valve (Lillehei-Kaster Medical, Inner Grove Heights, Minn.) was first issued in 1970. It was the first of the tilting disk valves to use a Pyrolite disk; the previous valves had all used Delrin as the disk material. The Omniscience and Omnicarbon valves were developed from the original Lillehei-Kaster valve. The Omniscience valve replaced the retaining rails with earlike guards, and the device was made with a much lower profile. The disk housing is made of titanium, and the disk is Pyrolite. The Omnicarbon valve is essentially identical to the Omniscience valve, but it contains a full Pyrolite housing and disk apparatus.
These valves remain in production today, and more than 100,000 of them have been inserted. In a 20-year review of their experience with the Omniscience valve, Misawa et al found that the rate of thromboembolic complication was only 0.8%, with an identical rate of hemorrhagic complication. Teijeira reported on 200 Omniscience valve replacements and also found that 12-year thromboembolism-free and anticoagulation-free rates were approximately 80%. Nonstructural prosthetic valve dysfunction rates were 0.6%. In another study, Edwards et al reported a risk of thromboembolism of 0.2% to 1.3% and a risk of systemic embolic events of 1.57% to 2.90% per patient-year. Reports of these valves have had varied results and have been the source of considerable debate in the literature.
Monostrut Valve
The Monostrut valve (Shiley) contains two struts that are connected by machine to the housing, to reduce the chance of strut fracture. In a comparative study with the Carbomedics bileaflet valve, investigators reported a 0.2% per year risk of thromboembolism and a 0.6% per year risk of anticoagulation-related bleeding. In this study of 200 patients, no difference was noted between the Monostrut tilting disk valve and the Carbomedics valve.
Bileaflet Valves
The general design of the bileaflet valve consists of two semicircular disks that rotate about struts in the valve housing. These valves are in theory more hemodynamically sound; however, they are also susceptible to backflow. They are also generally less thrombogenic and require a lower amount of anticoagulation. Several models of this type of valve are available, and the most important are reviewed here.
St. Jude Medical Valve
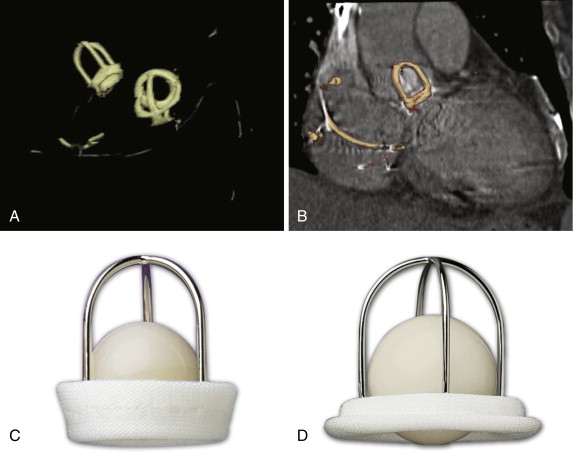
The St. Jude valves (St. Jude Medical, Inc., St. Paul, Minn) are the best known and most widely used of the bileaflet valves ( Figs. 17-6 and 17-7 ). Originally introduced in 1977, the valve consists of a Pyrolite housing with a sewing ring and Pyrolite hemidisks. The initial design concept was to have peripheral hinges. During the development stages, however, the inventors realized that this design was impractical, and the hinges were therefore moved to be more central in location. This configuration leaves a small central opening in the valve. To date, more than 1.3 million of these valves have been implanted, predominantly in the aortic and mitral valve positions.
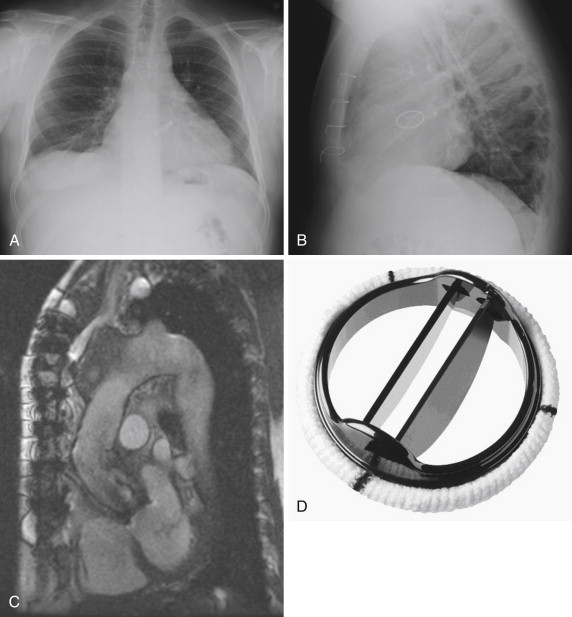
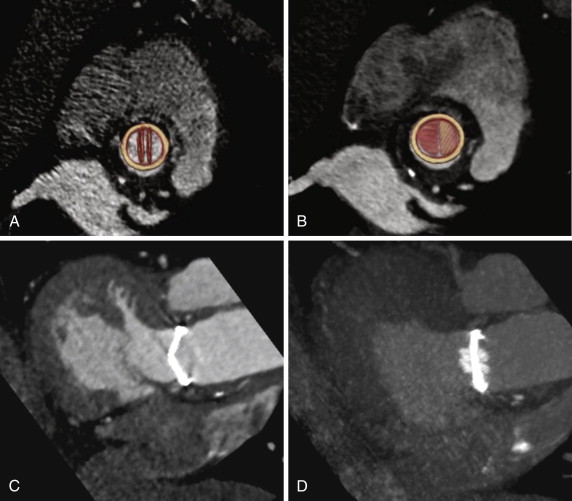
The safety profile of these valves has been extensively studied. In a 945-patient study, Toole et al reported their 25-year experience with 537 aortic valves and 408 mitral valves. In this follow-up study, in which the 5-,10-, 15-, and 20-year results had also previously been reported, all patients underwent single valve replacement. The study did not find a single structural failure of the valve. In the AVR group, 20% of all-cause mortality deaths were found to be valve related, of which bleeding and thromboembolism accounted for 78%, whereas in the MVR group, 14% of deaths were found to be valve related, of which bleeding and thromboembolism accounted for 89%. These findings translate to a 1.9% per patient-year risk of thromboembolism and a 3.0% per patient-year risk of bleeding in the AVR group. In the MVR group, the rate of thromboembolism was 3.2% per patient-year, and the rate of bleeding was 2.3% per patient-year.
In another large study by Khan et al, the main findings were that individuals with the St. Jude valve were significantly more likely to die as a result of non–valve-related complications, and 84% to 91% of these patients had not died of valve-related causes at 10 years. The overall survival was 42% to 43% at 10 years. In their study of 399 patients (4328 patient-years) with MVR, 471 with AVR, and 130 with double (mitral and aortic) valve replacement, thromboembolism rates were 2.4/100, 2.5/100, and 3.2/100 patient-years, for MVR, AVR, and double valve replacement, respectively, whereas the rates of hemorrhage were 1.9/100, 2.0/100, and 2.3/100 patient-years for MVR, AVR, and double valve replacements, respectively.
Another study by Remadi et al, reported in 2001, evaluated MVRs and found that actuarial survival (only from valve-related mortality) was 83% at 10- to 19-year follow-up. The overall survival rate in this group of 446 patients was 63%, a finding once again implying that many patients died of causes other than valve-related mortality. No structural dysfunction was observed during the follow-up period. The most frequent complications were related to anticoagulants, at 1% per patient-year, and the valve thrombosis rate was low, at 0.2% per patient-year.
Data are now also available for reoperation using the St. Jude valvular prosthesis for redo open heart surgery valve replacement. The two most common indications for redo open heart surgery are a new requirement for coronary artery bypass grafting and replacement of a previously implanted bioprosthetic valve. The operative morbidity of redo sternotomy, even in the best of hands, is higher than that of the primary sternotomy. If a third sternotomy is required, the risk increases even further; therefore, valve selection at the outset is of paramount importance. In their study, Emery et al demonstrated that in patients with redo sternotomy, the St. Jude valve continued to provide a low incidence of valve-related events. No valve-related structural degeneration was noted that would have led to a repeat replacement of the St. Jude valve, and thromboembolic- and anticoagulant- related hemorrhage remained the most important consideration influencing postoperative valve-related morbidity.
Noninvasive techniques for assessment of valve morphology and function are limited. Echocardiographic assessment of mechanical valvular prosthetic function using Doppler techniques to measure gradients has a role. This method can be combined with cinefluoroscopic evaluation to achieve a reasonable assessment of valvular mechanical function. The type of valvular prosthesis can often be ascertained using cinefluoroscopy. Furthermore, the disk opening angle can be assessed using cinefluoroscopy, which, in combination with transesophageal echocardiography for the detection of pannus, can be used to distinguish among structural failure, pannus formation, and valve-prosthesis mismatch.
Investigators are establishing gradient measurements by means of phase contrast MRI; however, normative value determination is still in the experimental stages. The role of CT imaging to establish valvular mechanical dysfunction is discussed in the later section on complications.
Carbomedics Valve
The Carbomedics valve (Carbomedics, Austin, Tex.) was introduced in 1986. Although this valve resembles the St. Jude valve in its construction, it has a housing that can be rotated within the sewing ring. Subsequent to the original design, the company designed a Top Hat Carbomedics valve that could be used for implantation in patients with small aortic roots. The modification in the Top Hat device involved only the sewing ring. To date, more than 500,000 Carbomedics valves have been implanted worldwide.
Long-term follow-up results of the Carbomedics valve are sparse. In a 10-year comparison study, Bryan et al demonstrated relative equivalency of the St. Jude valve and the Carbomedics valve and found that the Carbomedics valve had a 95% rate of freedom from valve-related mortality. The thromboembolism- and anticoagulation-related complication rates in this study were 1.1% and 2.3%, respectively, not statistically significantly different from the St. Jude valve. The Carbomedics valve also has the favorable hemodynamics expected from the bileaflet valve design.
In another study, Roedler et al demonstrated essentially no difference in valve-related complications between the traditional Carbomedics valve and the Top Hat valve. The same publication also mentioned the possibly advantageous features of the Sorin Mitroflow (Sorin Biomedica, Saluggia, Italy) valve. Kandemir et al also demonstrated results similar to those of the previously described studies and showed that both the St. Jude and Carbomedics valves had excellent intermediate-term results.
Sorin Bicarbon Valve
Introduced in 1990, the Sorin Bicarbon valve (Sorin Biomedica) represents another in the list of bileaflet valves. In a study of 1900 patients who were followed up to 15 years, Azarnoush et al confirmed a positive experience with this valve from their previously reported 5- and 10- year experience. The rate of valve-related thrombosis showed actuarial freedom at 15 years of 99.6% (98.6% to 99.9%) or 0.81% per patient-year. Hemorrhage related to anticoagulant treatment occurred in 293 cases, a finding that translated to 1.46% per patient-year, and the risk of endocarditis was 0.22% per patient-year. The hemodynamic profile of this valve has also shown to be favorable, much like the others in this category, with good LV mass regression.
Edwards MIRA Valve
The Edwards MIRA bileaflet valve (Edwards Lifesciences, Inc., Irvine, Calif) was introduced in 1998. It is composed of the main body of the Sorin mechanical prosthesis, with a swing ring that is comparable to that of the Starr prosthesis. Data on outcomes related to this valve are sparse; however, initial results of the Edwards MIRA valve also demonstrate a favorable risk profile. The design of the valve itself incorporates a thicker sewing ring than other valvular designs and thus offers better surgical visualization at the time of insertion. The valve also demonstrates favorable hemodynamics.
Bioprosthetic Valves
The ratio of mechanical to bioprosthetic valve implantation is approximately 55% to 45%, with the number of bioprosthetic valves increasing because of reduced risk of thrombogenicity and better durability than previous designs. Bioprosthetic valves can generally be classified into human-derived and animal-derived valves. Human-derived valves can be autografts or allografts. Autografts represent a patient’s native valve that is, in essence, transposed from one location to the other, as in the Ross procedure (pulmonary artery to aortic position, and artificial replacement of the less critical pulmonary artery). Allografts are harvested from cadaveric donors. Animal-derived valves are generally porcine aortic valves, or alternatively, they can be fashioned from bovine pericardial tissues. These grafts are often referred to as xenografts or heterografts.
Grafts can be both stented and stentless. Stented grafts are more common and employ a housing over which the appropriate valve can be fixed. The disadvantage of these systems is that the stent itself can compromise the valve orifice area. In response to this concern, stentless valves have been developed that can be sewn in without the housing present in the stented valves.
Stented Bovine Pericardial Valves
Carpentier-Edwards Perimount Valve
The Carpentier-Edwards bovine pericardial valve (Edwards Lifesciences, Irvine, Calif.) is a common bioprosthetic valve ( Figs. 17-8 and 17-9 ). This stented aortic valve prosthesis is made of bovine pericardium. The Carpentier-Edwards bovine pericardial valve was originally introduced in 1981; however, it did not become available clinically in the United States until 1991. The long-term hemodynamic performance of this valve has been well established, with effective long-term transvalvular gradients and no significant change in the orifice areas with time. At 17 years, approximately 13% of the patients with these valves have progressed to severe aortic regurgitation. In the short term, similar results have been found in the mitral position as well.