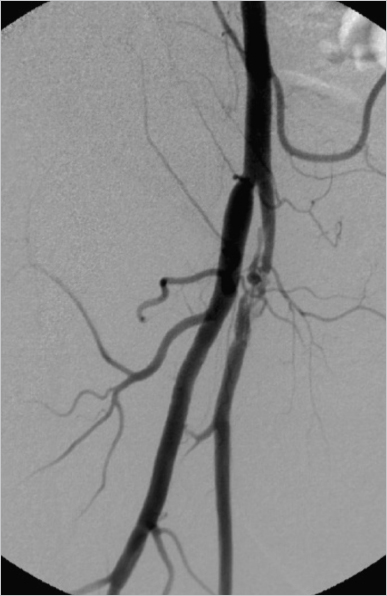
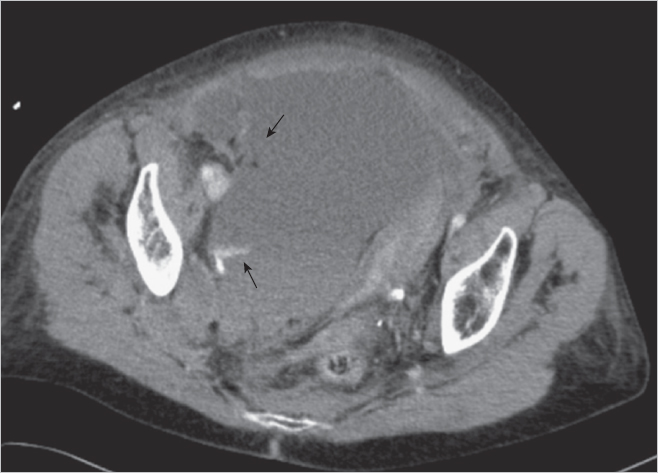
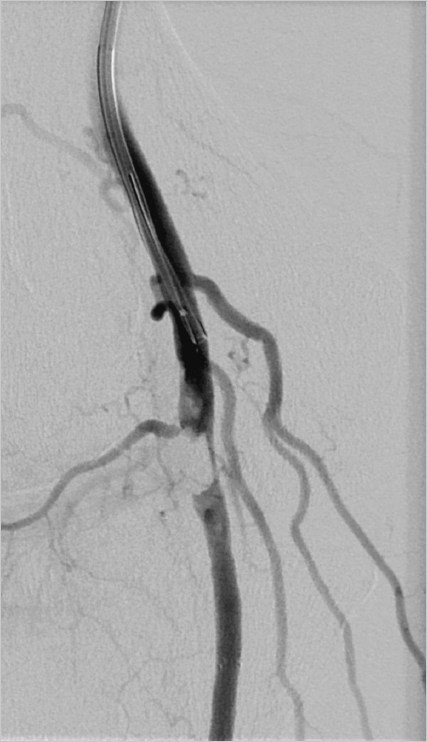
4 Case-Based Procedure-Related Complications A 64-year-old woman with peripheral vascular disease presented with acute limb ischemia of her left leg. Cardiovascular risk factors were hypertension, coronary heart disease, and smoking. She had mild rest pain; the leg was still viable (Rutherford IIa). It was decided to treat the patient endovascularly by locoregional lysis. Antegrade puncture of the left common femoral artery was performed. Selective angiography revealed acute occlusion of the superficial femoral artery (SFA) without visible reconstitution of flow in popliteal and infragenual arteries (Fig. 4.1). The patient was heparinized and a 10-cm side-hole catheter was placed into the proximal aspect of the SFA (Fig. 4.2). A bolus of 5 mg of recombinant tissue plasminogen activator (rtPA) was applied and long-term locoregional thrombolysis was initiated with 1 mg rtPA per hour. After 24 hours of lysis there was still significant thrombus burden; thus, locoregional treatment was continued for another 12 hours (total cumulative dose ca. 35 mg). Angiography after 36 hours showed residual thrombus in the below-the-knee (BTK) arteries (Fig. 4.3). In addition, there was wall-adherent clot present in the SFA. Residual clot was treated by aspiration and intra-arterial bolus application of 5 mg rtPA. Control angiography now showed an acceptable result with unobstructed flow in the SFA and a single infragenual artery reaching the foot (Fig. 4.4). Thrombolysis was stopped and the patient was continued on IV heparin (target partial thromboplastin time [PTT] 60–80 s). The sheath in the left groin was left in place for another 2 hours. It was then removed and the groin was manually compressed. After hemostasis was achieved a pressure bandage was applied and the patient transported to the clinical ward for further observation. During the first 24 hours of treatment the patient developed mild groin hematoma that started oozing from the puncture site. This problem was managed by changing the 4F sheath to a 6F standard sheath after 24 hours of lysis (Fig. 4.5). The remainder of the locoregional treatment time was clinically unremarkable. Fig. 4.3 Angiography after 36 hours of locoregional lysis showing residual thrombus in the BTK arteries (arrow). Fig. 4.4 Final result after 36 hours of locoregional lysis, bolus application of rtPA, and aspiration thrombectomy. Fig. 4.5 Following exchange of a 4F sheath with a 6F standard sheath no extravasation of contrast material was detected. Angiographic controls after 24 and 36 hours. Two hours after final sheath removal the patient was found lying unresponsive in her bed. There was only mild groin hematoma, which appeared to be unchanged from the previous episode of mild subcutaneous bleeding. Cardiopulmonary resuscitation was performed immediately and it was possible to stabilize the patient with a sinus rhythm. However, she remained in a coma and needed ventilation. Subsequently performed cranial MRI showed diffuse cortical hyperintensities indicating global hypoxemic damage to the brain (Fig. 4.6). The patient died 3 days after treatment. Autopsy showed a moderate retroperitoneal hematoma. • Evaluate alternatives for local fibrinolysis therapy as mechanical thrombectomy, eventually combined with low-dose fibrinolysis, especially in older patients. Fig. 4.6 Fluid-attenuated inversion-recovery (FLAIR) magnetic resonance imaging (MRI) shows cortical hyperintensities consistent with diffuse hypoxemic grey matter damage. • In case of local fibrinolysis therapy, check the patient carefully for potential bleeding complications. • All patients undergoing long-term fibrinolysis must be referred to an ICU. • Additional imaging, such as ultrasound and even native CT in clinical unclear situations, should be indicated immediately. An approach to control local bleeding was performed by upsizing the sheath from 4F to 6F. However, the retroperitoneal hematoma was unrecognized. Control of vital parameters as possible in an ICU is of utmost importance. There was unrecognized retroperitoneal bleeding leading to hypovolemia and consecutive cardiorespiratory arrest. This type of complication can only be managed by close clinical surveillance. In case of suspected retroperitoneal bleeding computed tomography (CT) should be performed. Signs of retroperitoneal bleeding in patients on locoregional lysis should prompt termination of the procedure. • Avoid antegrade punctures in patients with acute limb ischemia. Locoregional lysis should be performed preferably using a retrograde crossover approach, which limits the likelihood of significant bleeding complications. • Limit peripheral thrombolysis in terms of dose and time of treatment. Bleeding complications are more likely to occur the longer the patients are treated. • Use active closure device after local thrombolysis treatment. Rajan DK, Patel NH, Cardella JF et al. Quality improvement guidelines for percutaneous management of acute limb ischemia. J Vasc Interv Radiol 2009;20(7 suppl):208–218 Working Party on Thrombolysis in the Management of Limb Ischemia. Thrombolysis in the management of lower limb peripheral arterial occlusion—a consensus document. Am J Cardiol 1998;81(2):207–218 A 76-year-old patient suffered from lifestyle-limiting claudication (walking < 200 m). Noninvasive diagnostic imaging presented a moderate stenosis of the proximal right-side external iliac artery. She was scheduled for angioplasty via an ipsilateral retrograde groin approach for percutaneous transluminal angioplasty (PTA) or even stenting, if warranted. Ipsilateral retrograde groin (common femoral artery [CFA]) puncture under local anesthesia; successful insertion of a 4F 10-cm-long standard sheath. Development of ipsilateral groin swelling! Angiography of the right groin in an oblique projection to detect an underlying vessel injury. Mild contrast media extravasation was depicted, probably at the posterior wall of the CFA (Fig. 4.7). • Manual compression (MC) of the groin for at least 5 minutes, while leaving the sheath in place. • When MC fails, creation of contralateral access with crossover balloon tamponade at the level of the bleeding site; preparation of consecutive endovascular procedures, for example, coiling of side branches (if they seem to be the source of bleeding). • MC of the groin for at least 10 minutes after sheath removal. Fig. 4.7 Digital subtraction angiography (DSA). Contrast injection through the side port of the 4F sheath. DSA shows mild lateral contrast media extravasation (arrow) at the level of the lower third of the CFA. The source of bleeding was suspected to be at the lateral aspect of posterior wall. • Upsizing of 4F sheath until hemostasis (if leakage at the arteriotomy). • Surgery (if an endovascular approach fails). MC (5 minutes) of the groin with the sheath left in place. Control angiography showed no further bleeding (Fig. 4.8). Retrograde puncture of the CFA resulted in vessel wall damage. There was penetration of the posterior lateral side of the CFA wall resulting from the initial puncture with an open Seldinger needle. • In case of unclear anatomy of the CFA (i.e., obese patients, nonpalpable pulse), ultrasound should be used in order to gather further anatomical information. • Sufficient groin compression, even after failed puncture is of utmost importance! • Perform every vessel puncture, even retrograde access to the CFA, with great care even if it appears simple! • Be aware of groin swelling and hematoma formation at the puncture site; do not hesitate to look for a potential bleeding source, especially prior to further anticoagulation! • Prefer “anterior stick” puncture to classical Seldinger technique with intentional penetration of posterior femoral wall. Posterior vessel wall injuries may lead to significant bleeding complications and impair thrombolysis treatment for cases of distal embolization. Kalish J, Eslami M, Gillespie D, et al; Vascular Study Group of New England. Routine use of ultrasound guidance in femoral arterial access for peripheral vascular intervention decreases groin hematoma rates. J Vasc Surg 2015;61(5):1231–1238 Lo RC, Fokkema MT, Curran T, et al. Routine use of ultrasound-guided access reduces access site-related complications after lower extremity percutaneous revascularization. J Vasc Surg 2015;61(2):405–412 A 79-year-old patient presented with right groin hematoma and groin swelling 2 days after percutaneous coronary intervention via retrograde access to the right common femoral artery (CFA). Apart from his coronary heart disease no further comorbidities were reported. After coronary angiography a vascular closure device was used successfully and immediate hemostasis of the puncture site was achieved. The patient now presented in a hemodynamically stable condition. Clinical inspection of the right groin showed significant hematoma and swelling with a pulsating tumor. Percutaneous coronary intervention (PCI). Unremarkable procedure, no complications reported! Color duplex ultrasound of the right groin. There was a pseudoaneurysm located in the proximal aspect of the superficial femoral artery (SFA); in addition, an arteriovenous fistula (AVF) was depicted (Fig. 4.9). • Endovascular treatment via contralateral retrograde access to the CFA. • Catheterization of the feeder channel to the femoral vein, for example using a microcatheter and followed by selective microcoil embolization; however, the small aneurysm remains untreated. • Placement of a covered self-expanding stent (stent graft). • Surgery (if an endovascular approach fails). Endovascular treatment via contralateral retrograde access to the CFA (7F sheath, 45 cm long). A 0.035-inch guidewire placement in the SFA and stent graft placement covering both the fistula and the aneurysm (Figs. 4.10 and 4.11). Fig. 4.9 Angiography of the femoral artery bifurcation in crossover technique. A pseudoaneurysm is located in the proximal aspect of the SFA; initial femoral vein opacification is consistent with an AVF. Suspected (possibly repeated) retrograde puncture of the SFA resulted in vein and vessel wall penetration. Anticoagulation after the procedure and insufficient compression of the groin might have contributed to the development of this complication. Fig. 4.10 Oblique view during angiography showing the neck of the pseudoaneurysm, a nonflow-limiting dissection of the SFA, and the contrast filling of the femoral vein. A 6 × 10-mm e-PTFE (expanded polytetrafluoroethylene)-covered stent is already in place. • Avoid groin access with direct puncture of the SFA. • Sufficient groin compression, even after failed puncture, is of utmost importance! • If the location of the CFA is uncertain (i.e., obese patients, nonpalpable pulse), ultrasound should be used in order to gather further anatomical information. • Use the contralateral groin or an alternative access route (radial or brachial approach). Kendrick AS, Sprouse LR II. Repair of a combined femoral pseudoaneurysm and AVF using a covered stent graft. Am Surg 2007;73(3):227–229 A 65-year-old patient suffered from peripheral arterial occlusive disease (PAOD) with lifestyle-limiting walking distance of less than 150 m. Due to obesity, a right groin antegrade puncture of the common femoral artery (CFA) was chosen (part 1). Unfortunately, access failed and the patient developed minor groin hematoma resistant to manual compression. It was decided to finalize the procedure via crossover technique from a contralateral retrograde groin access (part 2). Initial diagnostic angiography (DA) showed some minor contrast extravasation at the recent puncture site. On top of that a distal embolic event occurred after initially planned PTA. Failed ipsilateral antegrade groin access (part 1). PTA of the femoropopliteal artery (part 2). Repeated failed CFA puncture (part 1). PTA of the femoropopliteal artery via crossover technique (part 2). Angiography. Mild contrast extravasation at the mid-part of the right CFA (Fig. 4.12). Acute occlusion of the posterior tibial artery located at the level of the medial malleolus (Figs. 4.13 and 4.14). Bleeding: • Endovascular treatment via contralateral retrograde access to the CFA. • Long-term PTA at the level of bleeding site. • Placement of a covered self-expanding stent (stent graft), which is usually not indicated at the CFA level. • Surgery (if an endovascular approach fails). Embolization: • Percutaneous aspiration thrombectomy (PAT). • Admission of local short-term fibrinolysis. • Surgery (rather unlikely at this distal level). Bleeding: Endovascular treatment via contralateral retrograde access to the CFA (6F sheath, 45 cm long). Long-term PTA for about 180 seconds at the bleeding level of the CFA using a 6 × 40-mm balloon (Figs. 4.15 and 4.16). Bleeding stopped after long-term PTA. Embolization: Four repetitions of PAT using a rapid-exchange (RX) 0.014-inch-compatible monorail catheter. The thromboembolism was sucked out successfully (Figs. 4.17–4.19). Bleeding: Repeated antegrade groin puncture caused vessel injury, which resulted in significant bleeding. Insufficient external compression during puncture might have contributed to the development of this complication. Embolization: Thromboembolism might have resulted from thrombus formation at the CFA and proximal superficial femoral artery (SFA), which is embolized down during PTA procedure. Bleeding: • Where the location of the CFA is uncertain (i.e., obese patients, nonpalpable pulse), a Doppler probe or B-mode ultrasound should be used in order to gather further anatomical information. • Sufficient groin compression, even after failed puncture, is of utmost importance! • Use the contralateral groin or an alternative access route (radial or brachial approach). • If ipsilateral access is essential, ask a colleague to assist you. Embolization: • Thromboembolism can be very unpredictable. Stone PA, Campbell JE. Complications related to femoral artery access for transcatheter procedures. Vasc Endovasc Surg 2012;46(8):617–623 Wu W, Hua S, Li Y, et al. Incidence, risk factors, treatment and prognosis of popliteal artery embolization in the superficial femoral artery interventions. PLoS One 2014;9(9):e107717 A 60-year-old patient suffering from recurrence of peripheral arterial occlusive disease (PAOD) was scheduled for diagnostic angiography (DA). During retrograde groin puncture there were problems inserting the atraumatic 0.035-inch sheath wire. A 0.035-inch hydrophilic wire was used to cannulate the artery through a Seldinger needle in order to establish access to the external iliac artery (EIA). The guidewire could be inserted but it could not easily be pulled back to correct the position. Despite considerable resistance the guidewire was removed through the needle, which was left in place. During this procedure scaling of the hydrophilic coating occurred, and the foreign material separated from the wire and remained inside the common femoral artery (CFA) (Fig. 4.20). Fig. 4.20 Scaled wire (Terumo) coating at the level of the origin of the superficial femoral artery (SFA). Note: a self-expanding stent is located in the proximal SFA. Failed retrograde groin access. Placement of a sheath wire prior to retrograde sheath placement failed. Attempts were made to insert a sheath over a hydrophylic-coated guide-wire. During removal of the wire, parts of the coating were scaled from the wire and remained in the vessel. Angiography from the contralateral groin. Iatrogenic foreign body in the CFA. • Endovascular treatment via contralateral retrograde access to the CFA. • Placement of a snare to engage the foreign body. • Surgery (if an endovascular approach fails). Endovascular treatment via contralateral retrograde access to the CFA was attempted (6F sheath, 45 cm long). Using a suture it was possible to grab the foreign body, which was finally removed (Figs. 4.21 and 4.22). Fig. 4.21 A snare was placed using crossover technique distally to the foreign body. The scaled wire fragment can be caught best by slightly rotating the open snare. Once the fragment is engaged and fixed, safe removal into the (crossover) sheath can be performed. Uncontrolled manipulation of a hydrophilic guidewire in combination with a sharp, beveled stainless steel puncture needle resulted in scaling of the coating. • Never pull a wire in an uncontrolled fashion through a beveled Seldinger needle. • If, during retrieval of a hydrophylic guidewire, resistance is felt, the wire should be removed together with the puncture needle. • Rotation of a deployed snare using a torque device might help catch a foreign body. Capuano F, Simon C, Roscitano A, Sinatra R. Percutaneous transluminal coronary angioplasty hardware entrapment: guidewire entrapment. J Cardiovasc Med (Hagerstown) 2008;9(11):1140–1141 Collins N, Horlick E, Dzavik V. Triple wire technique for removal of fractured angioplasty guidewire. J Invasive Cardiol 2007;19(8):E230–E234 Kang JH, Rha SW, Lee DI, et al. Successful retrieval of a fractured and entrapped 0.035-inch Terumo wire in the femoral artery using biopsy forceps. Korean Circ J 2012;42(3):201–204 Martí V, Markarian L. Angioplasty guidewire entrapment after stent implantation: report of two cases and review of the literature. Arch Cardiol Mex 2007;77(1):54–57 Ozkan M, Yokusoglu M, Uzun M. Retained percutaneous transluminal coronary angioplasty guidewire in coronary circulation. Acta Cardiol 2005;60(6):653–654 Rossi M, Citone M, Krokidis M, Varano G, Orgera G. Percutaneous retrieval of a guidewire fragment with the use of an angioplasty balloon and an angiographic catheter: the sandwich technique. Cardiovasc Interv Radiol 2013;36(6):1707–1710 An 83-year-old man was scheduled for diagnostic angiography (DA) for further evaluation of lower limb claudication. Both limbs were symptomatic. Failed ipsilateral retrograde groin access, therefore switching to right-side contralateral retrograde groin puncture for performing DA (Fig. 4.23). Due to failed placement of a sheath wire through the open Seldinger needle for further retrograde sheath placement, the operator switched to a hydrophilic-coated wire. Slight advancement of the wire was possible, but access to the true vessel lumen failed and the attempt to remove the wire back through the needle was only successful under considerable force. On examination, it was noticed that the coating was torn from the wire core (Fig. 4.24). Fig. 4.23 DA from the right groin after failed retrograde access via the left groin. The left common femoral artery is not narrowed. Angiography from the contralateral groin in crossover technique to visualize any ripped-off hydrophilic coating potentially compromising blood flow. Fig. 4.24 Photograph of the retrieved wire after failed placement. Note the separation of hydrophilic coating and wire core. Parts of the distal hydrophilic coating are missing. Intramural iatrogenic foreign body displacement of parts from hydrophylic coating. • No action was undertaken due to the extraluminal position of the torn coating and the uncompromised flow verified by DA. • In the case of an intraluminal position of the torn-offcoating, attempts at placement of a snare to engage the foreign body would be recommended. • Surgery (if an endovascular approach fails). Everything was left as it was due to the uncompromised flow and the extraluminal position of the coating (Figs. 4.25 and 4.26). The patient was informed of the problem. An uncontrolled manipulation of a hydrophilic guidewire in combination with a sharp stainless steel Seldinger needle resulted in snarling of the coating. • Never remove a wire in an uncontrolled manner back through a Seldinger needle. • If during guidewire manipulation a resistance is noticed, the wire with the entire needle needs to be removed in order to avoid any damage of the hydrophilic coating with resulting ripping and potential compromise of blood flow or embolization. Fig. 4.25 Magnified angiography indicating the extraluminal position of the separated hydrophylic wire coating. Kang JH, Rha SW, Lee DI, et al. Successful retrieval of a fractured and entrapped 0.035-inch Terumo wire in the femoral artery using biopsy forceps. Korean Circ J 2012;42(3):201–204 Patel T, Shah S, Pandya R, Sanghvi K, Fonseca K. Broken guidewire fragment: a simplified retrieval technique. Catheter Cardiovasc Interv 2000;51:483–486 Rossi M, Citone M, Krokidis M, Varano G, Orgera G. Percutaneous retrieval of a guidewire fragment with the use of an angioplasty balloon and an angiographic catheter: the sandwich technique. Cardiovasc Interv Radiol 2013;36(6):1707–1710 Van Gaal WJ, Porto I, Banning AP. Guidewire fracture with retained filament in the LAD and aorta. Int J Cardiol 2006;112:e9–e11 A 48-year-old man suffered from severe upper thigh pain that developed immediately after diagnostic coronary angiography via retrograde access to the right common femoral artery (CFA). Apart from his coronary heart disease no further comorbidities were reported. Hemostasis of the puncture site was achieved with manual compression. Clinical inspection showed right groin and upper thigh swelling, while clinical examination revealed a deep palpating mass and an upper thigh bruit. Diagnostic coronary angiography. Multiple punctures in order to gain right femoral artery access. Otherwise no obvious complications were encountered during the procedure. Color Doppler ultrasonography, followed by computed tomography angiography (CTA) if necessary. Reservation of digital subtraction angiography (DSA) through a contralateral femoral approach in case endovascular repair was considered. Deep femoral artery (DFA) pseudoaneurysm (PA) with extensive mural thrombus and small residual lumen (Fig. 4.27) in combination with ipsilateral arteriovenous fistula (AVF) between superficial femoral artery (SFA) and common femoral vein (CFV) (Fig. 4.28) verified by color Doppler ultrasound. • Close observation for smaller PAs and AVFs as there is a possibility of spontaneous closure. • Noninvasive procedures such as prolonged pressure bandaging and ultrasound-guided compression. • Percutaneous ultrasound-guided thrombin injection for the treatment of PAs. • Stent graft implantation mostly through percutaneous contralateral retrograde access to the CFA. • Catheterization of the feeder vessel, for example using a microcatheter and followed by selective microcoil embolization. • Alternative agents for embolization: glue, large particles, gelfoam. • Surgery (if endovascular approach fails). Compression to the right groin using the ultrasound probe for 30 minutes failed to seal both the AVF and the PA. Endovascular treatment via contralateral retrograde access to the CFA (8F 45-cm-long sheath) was attempted next. Selective right CFA and SFA angiograms confirmed the presence of a PA originating from a DFA branch in combination with an AVF between proximal SFA and CFV (Figs. 4.29 and 4.30). Fig. 4.28 Color Doppler ultrasound depicts a fistulous track between right SFA and CFV verified by a mixture of colors and increased Doppler waveform velocities. Initially a 6 × 40-mm self-expandable covered stent (Fluency, Bard PV) (Fig. 4.31) was deployed at the site of the AVF (Fig. 4.32), and subsequently the DFA branch from which the PA originated was superselectively catheterized with a 2.4F 130-cm-long coaxial microcatheter (Progreat, Terumo) (Fig. 4.33) and embolized with a 4-mm microcoil (Fig. 4.34). Before gaining proper femoral arterial access, multiple unsuccessful low femoral punctures were made, which resulted in perforation (with subsequent pseudoaneurysm development) of a DFA branch as well as AVF development between the SFA and CFV. Fig. 4.29 Selective right common femoral angiogram demonstrates the pseudoaneurysm originating from a DFA branch and the AVF between proximal SFA and CFV. • Avoid low femoral puncture, especially in obese patients in whom external landmarks for puncture (e.g., inguinal crease) can be misleading. • Ultrasound guidance for CFA access is strongly recommended in obese patients, in patients with weak femoral pulse, and following initial “blind” unsuccessful puncture attempts. • Color Doppler ultrasound should be the first imaging modality to detect all types of femoral arterial access complications in symptomatic patients. Fig. 4.31 Fluency self-expanding stent graft composed of a nitinol skeleton encapsulated with two ultrathin e-PTFE (expanded polytetrafluoroethylene) layers. Fig. 4.32 A 6 × 40-mm self-expandable Fluency covered stent was deployed that successfully sealed the AVF. Fig. 4.34 Final angiogram demonstrating successful sealing of both the pseudoaneurysm with a microcoil (arrow) and AVF by a stent graft. Deitch SG, Gupta R. Radioembolization complicated by dissection of the common femoral artery. Semin Interv Radiol 2011;28(2):133–136 Merriweather N, Sulzbach-Hoke LM. Managing risk of complications at femoral vascular access sites in percutaneous coronary intervention. Crit Care Nurs 2012;32(5):16–29 Tavris DR, Wang Y, Jacobs S, et al. Bleeding and vascular complications at the femoral access site following percutaneous coronary intervention (PCI): an evaluation of hemostasis strategies. J Invasive Cardiol 2012;24(7):328–334 An 82-year-old woman suffered from mild right groin hematoma and groin swelling, and onset of abdominal pain several hours after retrograde groin access for percutaneous coronary intervention (PCI) and final vascular closure with a 6F StarClose device (Abbott Vascular). Red blood cell count showed a significant decrease. The clinical condition of the woman declined significantly and contrast-enhanced computed tomography (CT) was initiated. PCI. Unremarkable procedure, no complications reported! Contrast-enhanced CT. A huge retroperitoneal hematoma was depicted in CT, reaching to the liver (Fig. 4.35) and showing contrast media extravasation at the right-side abdominal wall as an indicator of an active source of bleeding (Fig. 4.36). Questioning the operator for any problems during the recent PCI revealed that the patient had experienced an episode of heavy flank pain during hydrophilic guidewire advancement, which was self-limiting (after guidewire removal)! • Endovascular treatment via contralateral or ipsilateral retrograde access to the common femoral artery (CFA); angiography via the sheath/catheter to evaluate the exact origin of the bleeding. Preparation of selective catheterization of the feeder vessel, for example using a microcatheter, and followed by selective microcoil embolization distally and proximally to the bleeding source. Fig. 4.35 Axial contrast-enhanced CT scan showing the retroperitoneal hematoma reaching to the liver and the pararenal space. • Alternatives for embolization: glue, large particles, gelfoam. • Surgery (if an endovascular approach fails). Endovascular treatment via an ipsilateral retrograde access to the CFA (4F sheath, 10 cm long) was performed. After placement of a 2.7F microcatheter, the deep circumflex iliac artery was cannulated; contrast injection verified the bleeding source (Figs. 4.37 and 4.38). The microcatheter was placed beyond the bleeding source and two 4-mm coils were implanted (0.018-inch compatible). The coiling was completed by implanting additional coils proximally to the source of bleeding (Fig. 4.39). Fig. 4.36 Axial contrast-enhanced CT scan at the level of the anterior superior spine. A focal area of hyperdensity (arrow) is located at the inner side of the anterior oblique abdominal muscle. Fig. 4.37 Angiography of the right CFA and external iliac artery via the 4F sheath. The early phase depicts the deep circumflex iliac artery as the source of bleeding (arrow). Fig. 4.38 Angiography of the right CFA and external iliac artery via the 4F sheath. The later phase depicts a large area of contrast extravasation (arrow). Unrecognized hydrophilic-coated guidewire advancement into a hypogastric side branch resulted in vessel perforation and hemorrhage. Anticoagulation following the PCI procedure might have aggravated the extent of the bleeding. However, the fact that the operator ignored the sudden onset of self-limiting flank pain also contributed to the development of this complication. • Always visually control endovascular tools. Especially when using guidewires with a hydrophilic-coated tip, the distal position of the wire should be controlled by fluoroscopy and advanced with care. • If the patient reports sudden and unexpected pain, the operator should check his or her tools and rule out any complication; the patient is always right until proven otherwise! • Follow up your patients clinically; in case of any uncertainty, indicate further diagnostics! Kiviniemi T, Puurunen M, Schlitt A, et al. Performance of bleeding risk-prediction scores in patients with atrial fibrillation undergoing percutaneous coronary intervention. Am J Cardiol 2014;113(12):1995–2001 Lee MS, Applegate B, Rao SV, Kirtane AJ, Seto A, Stone GW. Minimizing femoral artery access complications during percutaneous coronary intervention: a comprehensive review. Catheter Cardiovasc Interv 2014;84(1):62–69 Mamas MA, Anderson SG, Carr M, et al. Baseline bleeding risk and arterial access site practice in relation to procedural outcomes after percutaneous coronary intervention. J Am Coll Cardiol 2014;64(15):1554–1564 Stone PA, Campbell JE, AbuRahma AF. Femoral pseudoaneurysms after percutaneous access. J Vasc Surg 2014;60(5):1359–1366 A 75-year-old patient with suspected bilateral carotid artery stenosis was referred for diagnostic angiography (DA). The patient was on low-dose aspirin and had received a loading dose of 300 mg of clopidogrel before the diagnostic procedure. None. Multiple attempts were made to puncture the right common femoral artery (CFA). However, access was created in the proximal aspect of the superficial femoral artery (SFA). Shortly after placement of a 5F standard sheath the patient presented clinical signs of hypovolemia (hypotension, dizziness, cold sweats). He also complained about pain in his right groin, which appeared tender and swollen. Angiography via the sheath in the right femoral artery was performed in order to detect possible vascular injury. There was active contrast extravasation close to the access site; however, the exact source was unclear (Fig. 4.40). • Upsizing sheath to account for possible leakage from sheath entry point. • Removal of the sheath and manual compression of the groin. • Antegrade access from contralateral groin in crossover technique; balloon tamponade, detection of bleeding source and further techniques (stent graft, embolization). • Surgery (if endovascular approach fails). Endovascular treatment via a left retrograde femoral access with crossover cannulation of the right iliac artery was initiated. Following a crossover manoeuver with a curved diagnostic catheter and a hydrophilic 0.035-inch guidewire, a 6F, 45-cm-long sheath was positioned with its tip in the external iliac artery. The guidewire was advanced into the SFA, the sheath was removed, and the proximal aspect of the vessel occluded for 5 minutes with a 6 × 40-mm standard balloon catheter. Control angiography via the crossover sheath detected continuous bleeding and identified a side branch of the deep femoral artery as the actual bleeding source (Fig. 4.41). The deep femoral artery was cannulated using a diagnostic catheter and a hydrophilic 0.035-inch guidewire. A 2.7F microcatheter was advanced through the diagnostic catheter into the bleeding side branch, which was embolized with platinum microcoils (Fig. 4.42). Postintervention no remaining extravasation was detected (Fig. 4.43). Fig. 4.41 Continuous contrast extravasation despite balloon tamponade of the arteriotomy, apparently due to injury of a side branch of the deep femoral artery. Multiple attempts to puncture the right CFA resulted in injury to a side branch of the deep femoral artery. Initial angiography via the sheath was misinterpreted and the actual injury was revealed after balloon tamponade of the arteriotomy was performed. Management of the complication was done endovascularly by microcoil embolization. Fig. 4.43 Control angiography following coil embolization of the bleeding side branch of the deep femoral artery. No extravasation is visible. • Limit the number of attempts to puncture the CFA. • When encountering difficulties use ultrasound guidance to gather further anatomical information and enable proper puncture. • Be aware of groin swelling and hematoma formation at the puncture site; do not hesitate to look for active bleeding, especially prior to further interventions. Bates MC, Campbell JE. Technique for ipsilateral rescue embolization of common femoral side branch vessel injury. Catheter Cardiovasc Interv 2007;70(6):791–794 A 63-year-old patient with right lower extremity intermittent claudication was referred to endovascular therapy for a distal stenosis of the right superficial femoral artery (SFA). Due to tortuous iliac artery anatomy an antegrade approach was chosen to address the lesion. SFA intervention. Initial puncture of the CFA was uneventful and a 6F standard sheath could be inserted without any problems. A few minutes after sheath placement the patient complained about dizziness, sweating, and nausea. There was no bleeding or hematoma visible; however, blood pressure measurements showed a significant pressure drop, indicating acute volume loss. Angiography via the sheath in the right femoral artery was performed in order to detect possible vascular injury. Angiography revealed a high common femoral puncture close to the origin of the inferior epigastric artery. There was contrast extravasation from the sheath entry point due to kinking of the sheath (Fig. 4.44). Apparently the bleeding was into the retroperitoneum, not the groin, due to the high entry point of the sheath. • Upsizing the sheath to seal leakage at the entry point. • Removal of the sheath and manual compression of the groin (least preferred option because bleeding was into the retroperitoneal space). • Antegrade access from contralateral groin using crossover technique and balloon tamponade. • Further endovascular treatment (stent graft, embolization). • Surgery (if an endovascular approach fails). To facilitate endovascular treatment of this complication the left femoral artery was punctured and retrograde femoral access was established. Following a crossover maneuver with a curved diagnostic catheter and a hydrophilic 0.035-inch guidewire, a 6F, 45-cm-long sheath was positioned with its tip in the external iliac artery. The guidewire was advanced into the SFA (Fig. 4.45), the kinked sheath was removed, and the puncture side occluded for 10 minutes with a 7 × 40-mm standard balloon catheter (Fig. 4.46). Control angiography via the crossover sheath did not show further bleeding (Fig. 4.47). High puncture of the right common femoral artery (CFA) in combination with vessel calcification led to kinking of the sheath. The arteriotomy was not sufficiently sealed, which resulted in retroperitoneal hemorrhage. Balloon tamponade was performed and hemostasis was reached without the need for further interventions. • With challenging groin anatomy, ultrasound guidance should be used to establish safe access to the CFA. • The puncture should aim at the mid-level of the CFA over the femoral head. • The puncture should aim at least 1 cm below the origin of the inferior epigastric artery to maintain a safe distance from the inguinal ligament and avoid retroperitoneal bleeding. • Significant and life-threatening hemorrhage can arise from the groin access even if there is no swelling or hematoma formation at the puncture site. Fig. 4.47 Control angiography following sheath removal and 10 minutes balloon tamponade does not show signs of ongoing bleeding. Seto AH, Abu-Fadel MS, Sparling JM, et al. Real-time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: FAUST (Femoral Arterial Access With Ultrasound Trial). JACC Cardiovasc Interv 2010;3(7):751–758 Vavuranakis M, Kalogeras K, Vrachatis D, et al. Inferior epigastric artery as a landmark for transfemoral TAVI. Optimizing vascular access? Catheter Cardiovasc Interv 2013;81(6):1061–1066 A 43-year-old patient with lifestyle-limiting claudication was referred for endovascular treatment of TASC (TransAtlantic Inter-Society Consensus) B-type lesion in the left superficial femoral artery (SFA). The patient was obese and had typical risk factors of peripheral vascular disease with hypertension, nicotine use, and hypercholesterolemia. None. Following local anesthesia the antegrade puncture of the left common femoral artery (CFA) was attempted. However, access to the artery was not reached and multiple punctures were tried. Before vessel access with wire insertion for sheath placement was established the patient started to complain of pain in the left groin. There was rapid development of tenderness and swelling in the left infrainguinal region. The patient also developed physiological signs of blood loss with development of hypotension and tachycardia, successfully controlled by leg elevation and fluid resuscitation. Injection of contrast medium through the puncture needle showed extravasation (Fig. 4.48). Retrograde puncture of the contralateral CFA, crossover cannulation, and sheath placement to the left iliac artery to locate the suspected bleeding source. There was active bleeding arising from the inferior epigastric artery due to a laceration (Fig. 4.49). • Manual compression of the groin. • Balloon tamponade. • Selective embolization. • Surgery (if an endovascular approach fails). Cannulation of the inferior epigastric artery and coil embolization (Figs. 4.50 and 4.51). In obese patients the skin entry point for an antegrade puncture often has to be above the inguinal ligament in order to establish high enough access to the CFA, which is needed for intubation of the SFA. In this case multiple attempts to puncture the left CFA failed and one of the high punctures resulted in laceration of the inferior epigastric artery. Manual compression was performed initially; however, it could not control the bleeding. A diagnostic catheter tracked over a hydrophylic guidewire using the crossover technique could easily cannulate the bleeding vessel. A single 3-mm platinum coil was sufficient to seal the lacerated artery and led to hemostasis. Fig. 4.49 Digital subtraction angiography showed laceration of the inferior epigastric artery and active bleeding. Fig. 4.51 Selective angiography showing the successfully embolized artery. No signs of continuous bleeding were present. • If the anatomy of the CFA is uncertain (i.e., obese patients, nonpalpable pulse), a Doppler probe or B-mode ultrasound should be used in order to gather further anatomical information. • When performing antegrade punctures, start as distally as possible and aim at the segment of the CFA over the femoral head. • Be aware of groin swelling and hematoma formation at the puncture site. Do not hesitate to look for potential bleeding sources, and be prepared for contralateral access. Park SW, Ko SY, Yoon SY, et al. Transcatheter arterial embolization for hemoperitoneum: unusual manifestation of iatrogenic injury to abdominal muscular arteries. Abdom Imaging 2011;36(1):74–78 Park YJ, Lee SY, Kim SH, Kim IH, Kim SW, Lee SO. Transcatheter coil embolization of the inferior epigastric artery in a huge abdominal wall hematoma caused by paracentesis in a patient with liver cirrhosis. Korean J Hepatol 2011;17(3):233–237 Sanchez CE, Helmy T. Percutaneous management of inferior epigastric artery injury after cardiac catheterization. Catheter Cardiovasc Interv 2012;79(4):633–637 Sobkin PR, Bloom AI, Wilson MW, et al. Massive abdominal wall hemorrhage from injury to the inferior epigastric artery: a retrospective review. J Vasc Interv Radiol 2008;19(3):327–332 Yalamanchili S, Harvey SM, Friedman A, Shams JN, Silberzweig JE. Transarterial embolization for inferior epigastric artery injury. Vasc Endovasc Surg 2008;42(5):489–493 In a 65-year-old man with buttock claudication, color duplex ultrasound detected common iliac artery (CIA) stenosis. The patient presented for interventional treatment. Apart from hypertension no further cardiovascular risk factors were present. None. Following local anesthesia in the left groin, retrograde puncture of the common femoral artery (CFA) was performed. During insertion of the introducer wire, resistance was felt; however, the guidewire was further advanced. During the maneuver the patient complained of moderate pain in the left pelvis/groin area. A standard 4F vascular sheath was inserted. Angiography. There was extensive dissection of the external iliac artery (EIA) reaching into the CFA (Fig. 4.52). Angiography revealed the position of the sheath to be subintimal (Fig. 4.53). The femoral pulse was still palpable and the patient did not present with any clinical signs of acute limb ischemia. Fig. 4.54 DSA 2 days after failed access and dissection showing restitution of the lumen and realignment of the intimal flap. • Crossover recanalization of the true lumen; angioplasty and consecutive stent placement (not preferred because of extension of the dissection into the CFA). • Abort procedure. Removal of the sheath and manual compression of the groin. Clinical re-evaluation. • Surgery (if endovascular approach fails). Because the femoral pulse in the left groin was palpable and no clinical signs of acute limb ischemia were present, it was decided to abort the procedure and remove the sheath. Manual compression was performed and hemostasis achieved. The patient was heparinized and rescheduled for angiography from the contralateral groin 2 days later. This follow-up study showed total restitution of the EIA lumen without need for further treatment (Fig. 4.54). The stenosis in the left CIA was successfully stented during this procedure. Guidewire advancement through the puncture needle was performed despite resistance. Apparently the sheath wire had entered the subintimal space and was further introduced, which dissected the EIA. Antegrade blood flow in the iliac artery was sufficient to realign the intimal flap to the vessel wall. Stent placement was not necessary. • Introduction of a sheath wire must be performed carefully. If any resistance is felt during wire advancement, the position of the puncture needle should be corrected until free backflow is visible and easy movement of the wire is possible. • In the case of uncertain needle location, contrast injections should be performed to rule out a subintimal position. • Use ultrasound for needle guidance for difficult femoral access. Kalish J, Eslami M, Gillespie D, et al; Vascular Study Group of New England. Routine use of ultrasound guidance in femoral arterial access for peripheral vascular intervention decreases groin hematoma rates. J Vasc Surg 2015;61(5):1231–1238. Lo RC, Fokkema MT, Curran T, et al. Routine use of ultrasound-guided access reduces access site-related complications after lower extremity percutaneous revascularization. J Vasc Surg 2015;61(2):405–412 Stone PA, Campbell JE. Complications related to femoral artery access for transcatheter procedures. Vasc Endovasc Surg 2012;46(8):617–623 Tsetis D. Endovascular treatment of complications of femoral arterial access. Cardiovasc Interv Radiol 2010;33(3):457–468 A 72-year-old man with a long history of peripheral vascular disease presented with worsening claudication in his left leg. A high-grade stenosis of the left common femoral artery (CFA) was detected by ultrasound. The vascular surgeon requested diagnostic angiography (DA) before reconstructive surgery. Status post right femoropopliteal bypass graft. Following local anesthesia in the right groin, retrograde puncture of the CFA was performed. Insertion of an introducer wire and placement of the 5F sheath were uneventful; however, angiography revealed extensive dissection of the right external iliac artery (EIA). During the procedure the patient developed rest pain in his right leg. Angiography. Extensive dissection of the right EIA (Fig. 4.55). • Crossover recanalization of the true lumen from a retrograde left groin approach; angioplasty and consecutive stent placement (not preferred because of high-grade stenosis in the left CFA). • Recanalization from a transbrachial approach; angioplasty and consecutive stent placement. • Abort procedure. Removal of the sheath and manual compression of the groin (not preferred because patient showed signs of acute limb ischemia). Fig. 4.55 Digital subtraction angiography (DSA) showing extensive dissection of the right EIA. Note the calcified plaque in the left EIA with high-grade stenosis. • Surgery (if an endovascular approach fails). Following retrograde puncture of the left brachial artery a 90-cm-long 6F sheath was positioned into the infrarenal aorta. The right iliac artery was recanalized with a diagnostic catheter and a 0.035-inch guidewire. Two self-expanding stents were implanted and postdilatated. Postinterventional angiography showed no residual stenosis or dissection (Fig. 4.56). Despite uneventful puncture and sheath insertion, iatrogenic dissection of the right iliac access occurred. This complication is more common in patients with heavily diseased peripheral arteries. It can be treated endovascularly from the contralateral groin or a transbrachial approach as shown in this case. • Iliac artery dissection from arterial groin access is rare, but potentially limb threatening. • Introduction of the sheath must be performed carefully. If any resistance is felt during wire placement the position of the puncture needle should be corrected until free advancement is possible. • If femoral access is difficult use ultrasound for needle guidance. • Hemodynamically relevant iatrogenic dissection should be treated. Kalish J, Eslami M, Gillespie D, et al; Vascular Study Group of New England. Routine use of ultrasound guidance in femoral arterial access for peripheral vascular intervention decreases groin hematoma rates. J Vasc Surg 2015;61(5):1231–1238 Lo RC, Fokkema MT, Curran T, et al. Routine use of ultrasound-guided access reduces access site-related complications after lower extremity percutaneous revascularization. J Vasc Surg 2015;61(2):405–412 Stone PA, Campbell JE. Complications related to femoral artery access for transcatheter procedures. Vasc Endovasc Surg 2012;46(8):617–623 Tsetis D. Endovascular treatment of complications of femoral arterial access. Cardiovasc Interv Radiol 2010;33(3):457–468 A 55-year-old man with claudication in his right leg presented for endovascular therapy. Noninvasive imaging showed a short-segment distal superficial femoral artery (SFA) occlusion. Planned SFA intervention using antegrade puncture technique. For unknown reasons a distal direct puncture of the SFA was performed. Following arterial backflow from the puncture needle a sheath guidewire was advanced. Despite resistance during wire advancement a 6F 45-cm-long sheath was inserted. Immediately after access, the patient complained of tenderness and pain in his right groin. Angiography via the sheath. Angiography showed the direct SFA puncture and acute rupture of the artery approximately 2 cm distally to the sheath entry point (Fig. 4.57). Note the very low entry point of the sheath (arrow) in relation to the femoral head (Fig. 4.58). The ostium of the sheath was close to the site of the rupture (Fig. 4.59). • Removal of the sheath and manual compression of the groin (not preferred because of distal sheath entry point and active bleeding). • Antegrade access to the right common femoral artery (CFA) from a left contralateral retrograde groin approach. • Further endovascular treatment (stent graft, embolization). • Surgery (if an endovascular approach fails). Endovascular treatment was performed from a left retrograde femoral approach. Following a crossover maneuver with a curved diagnostic catheter and a hydrophilic 0.035-inch guidewire, a 6F 45-cm-long sheath was positioned with its tip in the external iliac artery. The guidewire was advanced distally to the malplaced sheath. To stop the bleeding, a 6 × 60-mm balloon catheter was inflated covering both the sheath entry point and the vessel rupture (images not shown). The sheath was then removed. After 10 minutes there was still bleeding from the ruptured SFA; thus, stent graft placement was indicated. A 6 × 60-mm stent graft (Fluency, Bard PV) was implanted successfully stopping the bleeding (Fig. 4.60). Fig. 4.58 Fluoroscopy of the right groin showing the distal entry point of the vascular sheath (arrow) in relation to the femoral head (dotted line). Fig. 4.59 Digital subtraction angiography (DSA) of the right femoral artery showing contrast extravasation and sheath entry point (arrows). In this case two problems were encountered. First, the distal direct puncture of the right SFA was already a risk factor for the development of a pseudoaneurysm because the location cannot easily be controlled with manual compression. In addition—due to uncontrolled wire advancement—there was rupture of the proximal SFA with active bleeding that was not managed by internal tamponade. A stent graft long enough to cover both openings in the artery was used successfully to close the vessel injuries without further clinical sequelae for the patient. Fig. 4.60 Femoral DSA and diagnostic angiography after stent graft placement showing successful hemostasis. • In the case of challenging groin anatomy, ultrasound guidance should be used to ensure safe access to the CFA. • Femoral arterial puncture should aim at the mid-third of the femoral head at least 1 cm below the origin of the inferior epigastric artery. In this segment the artery can be compressed against the femoral head to achieve hemostasis. • The inguinal crease is an unreliable landmark when aiming at the CFA, especially in obese patients. • Direct SFA puncture should be avoided because it is not easily managed by manual compression. • Guidewires should not be advanced against resistance. • Always be prepared to access the contralateral groin to be able to detect and treat vessel injury from failed inguinal puncture. Gutzeit A, Graf N Schoch E, Sautter T, Jenelten R, Binkert CA. Ultrasound guided antegrade femoral access: comparison between the common femoral artery and the superficial femoral artery. Eur Radiol 2011;21(6):1323–1328 Kalish J, Eslami M, Gillespie D, et al; Vascular Study Group of New England. Routine use of ultrasound guidance in femoral arterial access for peripheral vascular intervention decreases groin hematoma rates. J Vasc Surg 2015;61(5):1231–1238 Lechner G, Jantsch H, Waneck R, Kretschmer G. The relationship between the common femoral artery, the inguinal crease, and the inguinal ligament: a guide to accurate angiographic puncture. Cardiovasc Interv Radiol 1988;11(3):165–169 Lee MS, Applegate B, Rao SV, Kirtane AJ, Seto A, Stone GW. Minimizing femoral artery access complications during percutaneous coronary intervention: a comprehensive review. Catheter Cardiovasc Interv 2014;84(1):62–69 Lo RC, Fokkema MT, Curran T, et al. Routine use of ultrasound-guided access reduces access site-related complications after lower extremity percutaneous revascularization. J Vasc Surg 2015;61(2):405–412 Merriweather N, Sulzbach-Hoke LM. Managing risk of complications at femoral vascular access sites in percutaneous coronary intervention. Crit Care Nurs 2012;32(5):16–29 Stone PA, Campbell JE. Complications related to femoral artery access for transcatheter procedures. Vasc Endovasc Surg 2012;46(8):617–623 Stone PA, Campbell JE, AbuRahma AF. Femoral pseudoaneurysms after percutaneous access. J Vasc Surg 2014;60(5):1359–1366 Tsetis D. Endovascular treatment of complications of femoral arterial access. Cardiovasc Interv Radiol 2010;33(3):457–468 A 62-year-old woman had undergone surgery (patch) of the common femoral artery (CFA) 6 weeks earlier. Upon presentation she still suffered from a limited walking distance (200 m) due to a moderate stenosis of the external iliac artery (EIA) above the inguinal ligament, which had been left untreated for unknown reasons during surgery. The patient was scheduled for endovascular treatment. Treatment plan included retrograde CFA puncture at the level of the patch, direct placement of a self-expanding stent, and closure of the puncture site using a vascular closure device. CFA patch; in secondary endovascular procedure direct placement of a self-expanding stent (SES) (8 × 60 mm) followed by percutaneous transluminal angioplasty (PTA) (7 × 60 mm) through a 6F sheath (Fig. 4.61). Closure of the puncture site was performed with a StarClose Vascular Closure System (Abbott Vascular). Removal of the activated closure device after step 1 (unfolding of the nitinol anchor) was a little bit rugged. However, the subsequent procedural steps were unremarkable. The device was successfully removed and hemostasis achieved. Fluoroscopy of the right groin including the EIA. Distraction, elongation, and dislocation of the distal part of the stent towards the entry level of the sheath/closure device (Fig. 4.62). • Endovascular management via contralateral groin access with placement of an additional stent using crossover technique. This is only possible if the disintegrated stent is located fully inside the vessel. • Surgical repair (if an endovascular approach fails). Fig. 4.62 Fluoroscopy of the distracted stent. The struts above and below the innominate line are distracted down towards the entry-level site at the anterior wall of the CFA patch. The distal stent markers are at different levels, indicating the complete disintegration of the distal stent struts. The complication was managed surgically because it was unclear whether parts of the stent had left the vessel through the puncture site. The vessel was visually inspected and the disrupted stent struts were fixed to the vessel wall by intraoperative stenting with an additional SES. In retrospect the endovascular approach using a crossover technique might also have been possible. Apparently, the nitinol anchor of the vascular closure device had engaged the distal end of the stent (Fig. 4.63). The stent struts probably got caught by the anchor so that removal of the system resulted in disintegration and dislocation of parts of the stent. • Carefully evaluate the level of vessel access in order to guarantee suitability for a vascular closure device (nondiseased, non–heavily calcified CFA, no deep femoral or superficial femoral artery puncture). • If the puncture level of the CFA is uncertain (i.e., obese patients, nonpalpable pulse), diagnostic angiography should be used in order to gather further anatomical information. • Insert and activate vascular closure devices under fluoroscopy to rule out any interaction between devices and stent (struts). • In the case of a known vascular implant near a puncture site check the correct position of the vascular closure device before activation. • Use manual compression instead of a closure device if safe application of the device cannot be guaranteed. • Use contralateral groin or an alternative access routes (radial or brachial approaches) in iliac procedures close inguinal ligament. Durack JC, Thor Johnson D, Fidelman N, Kerlan RK, LaBerge JM. Entrapment of the StarClose Vascular Closure System after attempted common femoral artery deployment. Cardiovasc Interv Radiol 2012;35(4):942–944 Johnson DT, Durack JC, Fidelman N, Kerlan RK, LaBerge JM. Distribution of reported StarClose SE vascular closure device complications in the manufacturer and user facility device experience database. J Vasc Interv Radiol 2013;24(7):1051–1056 Varghese R, Chess D, Lasorda D. Re-access complication with a Star-Close device. Catheter Cardiovasc Interv 2009;73(7):899–901 A 61-year-old man with limited walking distance of 100 m was treated successfully by angioplasty and stent placement in the distal superficial femoral artery (SFA). The patient had typical comorbidities (heavy smoker, hypertension, hyperlipidemia). Fig. 4.64 Angiography of the proximal SFA showing the entrance level of the 6F sheath at the mid-part of the CFA. Access route was via the ipsilateral common femoral artery (CFA) through a regular 6F sheath (Fig. 4.64). Groin puncture and treatment of index lesion was uneventful. Angioplasty (5 × 100 mm) was performed after passing the high-grade stenosis with an 0.018-inch hydrophilic-tip wire. Due to flow-limiting dissection with severe recoil, placement of a self-expanding stent (5 × 100 mm) was performed. Closure of the puncture site was managed with a vascular closure device (Angio-Seal, St. Jude Medical). Angio-Seal application was according to the instructions for use; however, during the pulling maneuver the suture tore apart. Because hemostasis was insufficient the procedure was completed by manual compression for 10 minutes. The next day the patient presented with intermittent pain at rest and a limited walking capacity of less than 10 m. Color duplex ultrasound for further evaluation. Dislocation of the entire Angio-Seal anchor with the collagen plug and parts of the suture into the mid-part of the SFA (Fig. 4.65). • Retrograde puncture of the contralateral groin. Placement of a long 6F sheath into the ipsilateral SFA using crossover technique. Try to perform a snaring maneuver of the dislodged Angio-Seal using a small snare or a stent retriever (originally intended for thromboembolism removal during acute stroke management). • Stenting of the iatrogenic stenosis using crossover technique. • Surgical removal of the dislodged Angio-Seal (if endovascular approach fails). Surgical cut-down of the ipsilateral groin and removal of the dislodged material using Fogarty maneuver. Closure devices with intraluminal anchor and collagen plug need a considerable amount of pulling force to fixate the anchor at the anterior vessel wall. This maneuver gives enough stability for pressing the collagen plug onto the vessel. In most cases successful sealing of the puncture site is achieved. In this case the Angio-Seal suture ruptured and parts of the closure device embolized into the SFA. This is more likely to happen with calcified plaque burden, which might be sharp enough to damage the suture, resulting in rupture. In this particular case the anchor may have been caught intraluminally by plaque material, leading to delivery of collagen material into the vessel. • Handle instruments with care. Pull the suture carefully but with enough force to completely seal by pushing the collagen down towards the outer anterior vessel wall while holding the suture tight. Fig. 4.65 Intraoperative angiography of the dislodged Angio-Seal anchor and plug in the proximal part of the SFA (arrow), which resulted in a severe, symptomatic high-grade stenosis. Applegate RJ, Sacrinty M, Kutcher MA, et al. Vascular complications with newer generations of Angio-Seal vascular closure devices. J Interv Cardiol 2006;19(1):67–74 Azmoon S, Pucillo AL, Aronow WS, et al. Vascular complications after percutaneous coronary intervention following hemostasis with the Mynx vascular closure device versus the Angio-Seal vascular closure device. J Invasive Cardiol 2010;22(4):175–178 Carey D, Martin JR, Moore CA, Valentine MC, Nygaard TW. Complications of femoral artery closure devices. Catheter Cardiovasc Interv 2001;52(1):3–7 Corley JA, Kasliwal MK, Tan LA, Lopes DK. Delayed vascular claudication following diagnostic cerebral angiography: a rare complication of the Angio-Seal arteriotomy closure device. J Cerebrovasc Endovasc Neurosurg 2014;16(3):275–280 Eggebrecht H, Von Birgelen C, Naber C, et al. Impact of gender on femoral access complications secondary to application of a collagen-based vascular closure device. J Invasive Cardiol 2004;16(5):247–250 Fargen KM, Velat GJ, Lawson MF, et al. Occurrence of angiographic femoral artery complications after vascular closure with Mynx and Angio-Seal. J Neurointerv Surg 2013;5(2):161–164 Klocker J, Gratl A, Chemelli A, Moes N, Goebel G, Fraedrich G. Influence of use of a vascular closure device on incidence and surgical management of access site complications after percutaneous interventions. Eur J Vasc Endovasc Surg 2011;42(2):230–235 Mukhopadhyay K, Puckett MA, Roobottom CA. Efficacy and complications of Angio-Seal in antegrade puncture. Eur J Radiol 2005;56(3):409–412 Prabhudesai A, Khan MZ. An unusual cause of femoral embolism: angioseal. Ann R Coll Surg Engl 2000;82(5):355–356 Suri S, Nagarsheth KH, Goraya S, Singh K. A novel technique to retrieve a maldeployed vascular closure device. J Endovasc Ther 2015;22(1):71–73 Thalhammer C, Joerg GR, Roffi M, Husmann M, Pfammatter T, Amann-Vesti BR. Endovascular treatment of Angio-Seal-related limb ischemia—primary results and long-term follow-up. Catheter Cardiovasc Interv 2010;75(6):823–827 Wille J, Vos JA, Overtoom TT, Suttorp MJ, Van de Pavoordt ED, De Vries JP. Acute leg ischemia: the dark side of a percutaneous femoral artery closure device. Ann Vasc Surg 2006;20(2):278–281 A 39-year-old woman was scheduled for percutaneous coronary intervention (PCI). PCI was successful and uneventful; a vascular closure device was used for management of vascular access. Retrograde 6F access at the level of the common femoral artery (CFA) was used for percutaneous transluminal angioplasty (PTA). The right groin access was closed with an Angio-Seal 6F (St. Jude Medical). Closure of the puncture site was successful and hemostasis initially achieved. Angio-Seal application was according to the instructions for use. No adverse events were noted. The next day prior to discharge, the patient reported a limited walking capacity of less than 50 m (ipsilateral, right-side lower limb). Color duplex ultrasound for further evaluation. Additional computed tomography angiography (CTA) was performed. Severe short-segment stenosis was depicted at the puncture site level of the CFA (Figs. 4.66–4.68). The Angio-Seal anchor, probably including the plug and parts of the suture, caused a severe stenosis. Fig. 4.66 CTA at the level of the CFA (coronal reconstruction). The right CFA is not significantly filled with contrast media (arrow), whereas the contralateral side is visible (also a main deep femoral artery branch). • Endovascular management with stent placement (con: flexion zone, near femoral bifurcation). • Open surgical groin access with removal of foreign body and reconstruction of the CFA. Surgical cut-down of the ipsilateral groin and removal of the Angio-Seal material and finalizing open repair with patch plasty (Fig. 4.69). Fig. 4.68 CTA (volume-rendering technique) at the level of the CFA. The right CFA is not significantly filled with contrast media, whereas the contralateral side appears uncompromised. Closure devices with intraluminal anchor and collagen plug need a considerable amount of pulling force to fixate the anchor to the anterior vessel wall. This maneuver is necessary to stabilize the anchor before pressing the collagen plug onto the vessel. In the current case the anchor and parts of the collagen plug were found inside the vessel lumen. This complication is possible when the anchor is caught by plaque material inside the vessel so that delivery of the collagen material can end up in the vessel lumen. In the present case no plaque or underlying CFA stenosis was visible. The initial pulling maneuver of the suture was probably insufficient so that collagen material was delivered to the anchor still located inside the vessel and away from the puncture site. • Handle instruments with care. Pull the suture carefully, but with enough force to achieve complete sealing when pushing the collagen down towards the outer anterior vessel wall. • When a vascular closure device is used patients should be evaluated clinically and/or by Doppler ultrasound before they are discharged to rule out complications. Applegate RJ, Sacrinty M, Kutcher MA, et al. Vascular complications with newer generations of Angio-Seal vascular closure devices. J Interv Cardiol 2006;19(1):67–74 Azmoon S, Pucillo AL, Aronow WS, et al. Vascular complications after percutaneous coronary intervention following hemostasis with the Mynx vascular closure device versus the Angio-Seal vascular closure device. J Invasive Cardiol 2010;22(4):175–178 Carey D, Martin JR, Moore CA, Valentine MC, Nygaard TW. Complications of femoral artery closure devices. Catheter Cardiovasc Interv 2001;52(1):3–7 Corley JA, Kasliwal MK, Tan LA, Lopes DK. Delayed vascular claudication following diagnostic cerebral angiography: a rare complication of the Angio-Seal arteriotomy closure device. J Cerebrovasc Endovasc Neurosurg 2014;16(3):275–280 Eggebrecht H, Von Birgelen C, Naber C, et al. Impact of gender on femoral access complications secondary to application of a collagen-based vascular closure device. J Invasive Cardiol 2004;16(5):247–250 Fargen KM, Velat GJ, Lawson MF, et al. Occurrence of angiographic femoral artery complications after vascular closure with Mynx and Angio-Seal. J Neurointerv Surg 2013;5(2):161–164 Klocker J, Gratl A, Chemelli A, Moes N, Goebel G, Fraedrich G. Influence of use of a vascular closure device on incidence and surgical management of access site complications after percutaneous interventions. Eur J Vasc Endovasc Surg 2011;42(2):230–235 Mukhopadhyay K, Puckett MA, Roobottom CA. Efficacy and complications of Angio-Seal in antegrade puncture. Eur J Radiol 2005;56(3):409–412 Prabhudesai A, Khan MZ. An unusual cause of femoral embolism: angioseal. Ann R Coll Surg Engl 2000;82(5):355–356 Thalhammer C, Joerg GR, Roffi M, Husmann M, Pfammatter T, Amann-Vesti BR. Endovascular treatment of Angio-Seal-related limb ischemia—primary results and long-term follow-up. Catheter Cardiovasc Interv 2010;75(6):823–827 Wille J, Vos JA, Overtoom TT, Suttorp MJ, Van de Pavoordt ED, De Vries JP. Acute leg ischemia: the dark side of a percutaneous femoral artery closure device. Ann Vasc Surg 2006;20(2):278–281 A 78-year-old woman with peripheral vascular disease was treated with stenting of the external iliac artery (EIA) for moderate calcified stenosis. The intervention was performed via retrograde access through the common femoral artery (CFA). Closure of the puncture site was done with a 6F Angio-Seal (St. Jude Medical) and hemostasis was achieved immediately. Because there was lack of clinical improvement with an unchanged walking distance of 200 m, the patient was scheduled for additional percutaneous transluminal angioplasty (PTA) of a moderate tandem stenosis located in the mid-part and distal part of the ipsilateral superficial femoral artery (SFA) 2 weeks after the index procedure. Retrograde 6F access at the level of the CFA for EIA stenosis. Closure of the puncture site was performed with Angio-Seal 6F. Angio-Seal application was according to instructions for use. No adverse events were noted. Hemostasis was achieved. Antegrade puncture of the CFA was done under local anesthesia, and a 6F sheath was placed into the mid-part of the CFA. For digital subtraction angiography (DSA), contrast medium was hand injected through the sheath. High-grade CFA stenosis due to intravascular location of the retrograde Angio-Seal closure (Fig. 4.70). The Angio-Seal anchor, probably including the plug and parts of the suture, caused a severe stenosis, which was even more pronounced by added obstruction from the sheath. • Endovascular management with crossover stent placement (con: flexion zone, near femoral bifurcation). • Open surgical groin access with removal of foreign body and reconstruction of the CFA. Surgical cut-down of the ipsilateral groin and removal of the Angio-Seal material. Closure devices with intraluminal anchor and collagen plug need a considerable amount of pulling force to fixate the anchor to the anterior vessel wall. This maneuver is necessary to stabilize the anchor before pressing the collagen plug onto the vessel. In the current case the anchor and parts of the collagen plug were found inside the vessel lumen. This complication is possible when the anchor is caught by plaque material inside the vessel so that delivery of the collagen material can end up in the vessel lumen. In this case no plaque or underlying CFA stenosis were visible. The initial pulling maneuver of the suture was probably insufficient so that collagen material was delivered to the anchor still located inside the vessel and away from the puncture site. Fig. 4.70 Angiography of the CFA and the SFA showing a high-grade stenosis resulting from the retrograde Angio-Seal closure. • Handle instruments with care. Pull the suture carefully, but with enough force to achieve complete sealing when pushing the collagen down towards the outer anterior vessel wall. • When a vascular closure device is used, patients should be evaluated clinically and/or by Doppler ultrasound before they are discharged to rule out complications. Applegate RJ, Sacrinty M, Kutcher MA, et al. Vascular complications with newer generations of Angio-Seal vascular closure devices. J Interv Cardiol 2006;19(1):67–74 Azmoon S, Pucillo AL, Aronow WS, et al. Vascular complications after percutaneous coronary intervention following hemostasis with the Mynx vascular closure device versus the Angio-Seal vascular closure device. J Invasive Cardiol 2010;22(4):175–178 Carey D, Martin JR, Moore CA, Valentine MC, Nygaard TW. Complications of femoral artery closure devices. Catheter Cardiovasc Interv 2001;52(1):3–7 Corley JA, Kasliwal MK, Tan LA, Lopes DK. Delayed vascular claudication following diagnostic cerebral angiography: a rare complication of the Angio-Seal arteriotomy closure device. J Cerebrovasc Endovasc Neurosurg 2014;16(3):275–280 Eggebrecht H, Von Birgelen C, Naber C, et al. Impact of gender on femoral access complications secondary to application of a collagen-based vascular closure device. J Invasive Cardiol 2004;16(5):247–250 Fargen KM, Velat GJ, Lawson MF, et al. Occurrence of angiographic femoral artery complications after vascular closure with Mynx and Angio-Seal. J Neurointerv Surg 2013;5(2):161–164 Klocker J, Gratl A, Chemelli A, Moes N, Goebel G, Fraedrich G. Influence of use of a vascular closure device on incidence and surgical management of access site complications after percutaneous interventions. Eur J Vasc Endovasc Surg 2011;42(2):230–235 Mukhopadhyay K, Puckett MA, Roobottom CA. Efficacy and complications of Angio-Seal in antegrade puncture. Eur J Radiol 2005;56(3):409–412 Prabhudesai A, Khan MZ. An unusual cause of femoral embolism: Angio-Seal. Ann R Coll Surg Engl 2000;82(5):355–356 Thalhammer C, Joerg GR, Roffi M, Husmann M, Pfammatter T, Amann-Vesti BR. Endovascular treatment of Angio-Seal-related limb ischemia—primary results and long-term follow-up. Catheter Cardiovasc Interv 2010;75(6):823–827 Wille J, Vos JA, Overtoom TT, Suttorp MJ, Van de Pavoordt ED, De Vries JP. Acute leg ischemia: the dark side of a percutaneous femoral artery closure device. Ann Vasc Surg 2006;20(2):278–281 A 67-year-old woman underwent percutaneous transluminal angioplasty (PTA) and superficial femoral artery (SFA) stent placement via antegrade access to the common femoral artery (CFA). After successful treatment, a vascular closure device was used to achieve immediate hemostasis of the puncture site. Uneventful PTA and SFA stent placement with application of a 6F ExoSeal vascular closure device (Cordis/Johnson & Johnson). The closure device was used according to instructions for use. However, loading of the device, gradual removal of the standard 6F sheath, and activation of the ExoSeal plug seemed a little difficult with a lot of friction. After activation and sheath/ExoSeal removal no hemostasis was achieved, even after 5 minutes of additional manual compression. Manual compression was needed for another 10 minutes until complete hemostasis was achieved. Color duplex ultrasound of the groin. Embolization of the collagen plug into the common femoral arterial lumen, resulting in severe obstruction (Fig. 4.71). Fig. 4.71 B-mode ultrasound showing the collagen plaque (arrow) inside the vessel lumen instead of outside of the anterior vessel wall. • Endovascular management with crossover stent placement (con: high material burden, femoral flexion zone, near femoral bifurcation). • Open surgical groin access with removal of foreign body and reconstruction of the CFA. This complication was resolved by surgically inspecting the vessel and removal of the collagen plug. An endovascular approach using crossover technique with snaring of the plug or stenting might have been possible, but bore the risk of downstream embolization or other complications. The surgical approach for resolving this complication was deemed more appropriate. A severely stenosed CFA at the entrance level of the sheath (Fig. 4.72) probably contributed to this complication. Due to the resulting friction forces during ExoSeal placement and activation, there was misplacement of the plaque into the vessel lumen. • Evaluate vessel access carefully in order to guarantee suitability for a vascular closure device (nondiseased, non–heavily calcified CFA, no deep femoral or SFA puncture). • When experiencing unexpected friction or other irregularities while applying a closure device, consider aborting the procedure and do manual compression instead. Kara K, Mahabadi AA, Rothe H, et al. Safety and effectiveness of a novel vascular closure device: a prospective study of the ExoSeal compared to the Angio-Seal and ProGlide. J Endovasc Ther 2014;21(6):822–828 A 75-year-old woman presented with new onset of claudication in her right leg following diagnostic cardiac catheterization 2 weeks earlier. Color Doppler ultrasound showed a short-segment high-grade stenosis of the right superficial femoral artery (SFA). Significant comorbidities were arterial hypertension, coronary heart disease, and diabetes. Status post diagnostic cardiac catheterization via retrograde 7F access in the right groin. Closure of the puncture site was performed with Angio-Seal 6F (St. Jude Medical). The procedure report did not note any problems during the procedure or after puncture site closure. Hemostasis had been reached using the vascular closure device; no bleeding complications in the groin were documented. Retrograde puncture of the contralateral common femoral artery (CFA) using the crossover maneuver and placement of a 45-cm-long 6F sheath into the right external iliac artery (EIA). Digital subtraction angiography with contrast medium injections through the sheath. There was dissection and high-grade SFA stenosis, most likely due to intravascular location of parts of the Angio-Seal applied 2 weeks earlier (Fig. 4.73). The angiogram also revealed that the initial puncture was not in the CFA, but located directly in the SFA. • Endovascular management with recanalization and crossover stent placement. • Endovascular management with primary atherectomy. • Open surgical groin access with removal of foreign body and reconstruction of the femoral artery. Endovascular management with recanalization and crossover stent placement (Figs. 4.74 and 4.75). Percutaneous vascular closure devices, especially when designed with an intraluminal component, may lead to iatrogenic vessel stenosis. Direct puncture of the SFA—as had been done here—is also associated with a greater likelihood of complications. Due to the relatively small vessel lumen of the SFA and the distance between skin level and the puncture site, malfunctioning of a vascular closure device is not uncommon in this situation. In this case, because of the distal location of the resulting stenosis, percutaneous endovascular management with stent placement solved the problem. Fig. 4.73 Angiography of right femoral artery revealed a dissection and high-grade stenosis in the proximal aspect of the SFA, most likely resulting from the Angio-Seal device placed 2 weeks earlier. • When a vascular closure device is used, patients should be evaluated clinically and/or by Doppler ultrasound before they are discharged to rule out complications. • In case of challenging groin anatomy, ultrasound guidance should be used to establish safe access to the CFA. • The puncture should always be aimed at the mid-level of the CFA over the femoral head. • Do not use vascular closure devices in small access vessels. Fig. 4.75 Following stent implantation and mild balloon angioplasty no residual stenosis was detected. Applegate RJ, Sacrinty M, Kutcher MA, et al. Vascular complications with newer generations of Angio-Seal vascular closure devices. J Interv Cardiol 2006;19(1):67–74 Azmoon S, Pucillo AL, Aronow WS, et al. Vascular complications after percutaneous coronary intervention following hemostasis with the Mynx vascular closure device versus the Angio-Seal vascular closure device. J Invasive Cardiol 2010;22(4):175–178 Carey D, Martin JR, Moore CA, Valentine MC, Nygaard TW. Complications of femoral artery closure devices. Catheter Cardiovasc Interv 2001;52(1):3–7 Corley JA, Kasliwal MK, Tan LA, Lopes DK. Delayed vascular claudication following diagnostic cerebral angiography: a rare complication of the Angio-Seal arteriotomy closure device. J Cerebrovasc Endovasc Neurosurg 2014;16(3):275–280 Eggebrecht H, Von Birgelen C, Naber C, et al. Impact of gender on femoral access complications secondary to application of a collagen-based vascular closure device. J Invasive Cardiol 2004;16(5):247–250 Fargen KM, Velat GJ, Lawson MF, et al. Occurrence of angiographic femoral artery complications after vascular closure with Mynx and Angio-Seal. J Neurointerv Surg 2013;5(2):161–164 Klocker J, Gratl A, Chemelli A, Moes N, Goebel G, Fraedrich G. Influence of use of a vascular closure device on incidence and surgical management of access site complications after percutaneous interventions. Eur J Vasc Endovasc Surg 2011;42(2):230–235 Mukhopadhyay K, Puckett MA, Roobottom CA. Efficacy and complications of Angio-Seal in antegrade puncture. Eur J Radiol 2005;56(3):409–412 Prabhudesai A, Khan MZ. An unusual cause of femoral embolism: Angio-Seal. Ann R Coll Surg Engl 2000;82(5):355–356 Thalhammer C, Joerg GR, Roffi M, Husmann M, Pfammatter T, Amann-Vesti BR. Endovascular treatment of Angio-Seal-related limb ischemia—primary results and long-term follow-up. Catheter Cardiovasc Interv 2010;75(6):823–827 Wille J, Vos JA, Overtoom TT, Suttorp MJ, Van de Pavoordt ED, De Vries JP. Acute leg ischemia: the dark side of a percutaneous femoral artery closure device. Ann Vasc Surg 2006;20(2):278–281 A 74-year-old woman following diagnostic cardiac catheterization was referred to the vascular surgery department for open repair of a pseudoaneurysm of the common femoral artery (CFA) (Fig. 4.76). Hours before the scheduled operation the patient developed signs of peripheral circulatory failure and needed fluid resuscitation. Diagnostic cardiac catheterization had been performed. Access closure was with manual compression. Postprocedure development of a pseudoaneurysm of the right femoral artery. Computed tomography angiography (CTA) to evaluate extent of groin aneurysm and identification of active bleeding. CTA confirmed diagnosis of a pseudoaneurysm in the right groin. However, in addition there was a huge expanding retroperitoneal hematoma of the pelvis with multiple small diffuse active bleeding sites (Fig. 4.77). • Endovascular management (embolization with coils, glue or collagen sponge). • Medical treatment with transfusion of blood cells and/or replacement of clotting factors. • Surgical repair (if endovascular management fails). The patient was transferred to the Angio Suite. The left CFA was punctured and a 45-cm-long 6F sheath was placed into the right common iliac artery (CIA). The right internal iliac artery (IIA) was cannulated using a 4F diagnostic catheter. Selective angiography revealed multiple small bleeding spots in the distal collateral network of the IIA (Figs. 4.78a, b and 4.79). For embolization, a gelfoam sponge slurry with contrast medium was prepared and injected into the IIA until complete stasis was achieved (Fig. 4.80). In this case a pseudoaneurysm of the CFA apparently progressed to a retroperitoneal hematoma. Because no single bleeding source was identified, the active bleeding was likely due to expansion of the groin hematoma with consecutive rupture of multiple smaller arteries. With ongoing hemorrhage, disseminated intravascular coagulation (DIC) developed, preventing hematoma from compressing the arterial feeders. In this situation temporary nonselective embolization of the IIA with gelfoam sponge stopped the active bleeding and worsening of hemodynamic compromise. Two days after successful embolization and consolidation of coagulation parameters the hematoma was removed and the pseudoaneurysm closed surgically. The patient fully recovered. Fig. 4.78 (a,b) Selective angiogram of the right IIA. No major bleeding arteries are identified, unless small side branch bleeding is identified (arrows). • Be aware of potentially fatal retroperitoneal progression of a pseudoaneurysm previously contained in the groin. • Be familiar with endovascular embolization techniques. • In a patient with a compromised coagulation system, primary operative management of diffuse pelvic bleeding should be avoided. Cale L, Constantino R. Strategies for decreasing vascular complications in diagnostic cardiac catheterization patients. Dimens Crit Care Nurs 2012;31(1):13–17 Görich J, Brambs HJ, Allmenröder C, et al. The role of embolization treatment of acute hemorrhage. Rofo 1993;159(4):379–387. [In German] Merriweather N, Sulzbach-Hoke LM. Managing risk of complications at femoral vascular access sites in percutaneous coronary intervention. Crit Care Nurs 2012;32(5):16–29 Stone PA, Campbell JE. Complications related to femoral artery access for transcatheter procedures. Vasc Endovasc Surg 2012; 46(8):617–623 Stone PA, Campbell JE, AbuRahma AF. Femoral pseudoaneurysms after percutaneous access. J Vasc Surg 2014;60(5):1359–1366 Tavris DR, Wang Y, Jacobs S, et al. Bleeding and vascular complications at the femoral access site following percutaneous coronary intervention (PCI): an evaluation of hemostasis strategies. J Invasive Cardiol 2012;24(7):328–334 Velmahos GC, Chahwan S, Hanks SE, et al. Angiographic embolization of bilateral internal iliac arteries to control life-threatening hemorrhage after blunt trauma to the pelvis. Am Surg 2000;66(9):858–862 Wiley JM, White CJ, Uretsky BF. Noncoronary complications of coronary intervention. Catheter Cardiovasc Interv 2002;57(2):257–265 Xu JQ. Effectiveness of embolization of the internal iliac or uterine arteries in the treatment of massive obstetrical and gynecological hemorrhages. Eur Rev Med Pharmacol Sci 2015;19(3):372–374 A 68-year-old man underwent coronary angiography via retrograde access to the right common femoral artery (CFA). A vascular closure device was used to achieve hemostasis of the puncture site. A few days after the procedure, the patient was readmitted with new onset of claudication in his right leg. Clinical examination was unremarkable. Uneventful coronary angiography with application of a 6F Angio-Seal vascular closure device (St. Jude Medical). The closure device was used according to the instructions for use. Hemostasis was achieved immediately. Color duplex ultrasound of the groin; computed tomography angiography (CTA). There was high-grade stenosis of the CFA allegedly due to an intravascular position of the collagen plug and anchor complex (Fig. 4.81). • Endovascular management with stent placement (con: high material burden, flexion zone). • Open surgical groin access with removal of foreign body and patch reconstruction. This complication was solved surgically. Intraoperative angiography (Fig. 4.82) confirmed high-grade stenosis from the foreign body. Surgery revealed that the collagen plug on the intravascular anchor had been placed partly inside the lumen. The foreign body was removed (Fig. 4.83) and the vessel reconstructed by patch plasty. Fig. 4.81 Axial image from CTA at the level of the puncture. Note high-grade stenosis in the right CFA. Fig. 4.82 Intraoperative angiography of the high-grade stenosis in the right CFA after Angio-Seal application. The reason for this complication remains unclear. During Angio-Seal placement the anchor probably became attached to a plaque in the CFA. When pushing the collagen plug down, the material must have entered the vessel lumen, leading to an intravascular position of the device. • Evaluate vessel access carefully in order to guarantee suitability for a vascular closure device (nondiseased, non–heavily calcified CFA, no deep femoral or superficial femoral artery puncture). • When experiencing unexpected friction or other irregularities while applying a closure device consider aborting the procedure and doing manual compression instead. • Carefully follow the instructions for use and do not hesitate to ask a colleague if something is unclear. Carey D, Martin JR, Moore CA, Valentine MC, Nygaard TW. Complications of femoral artery closure devices. Catheter Cardiovasc Interv 2001;52(1):3–7 Corley JA, Kasliwal MK, Tan LA, Lopes DK. Delayed vascular claudication following diagnostic cerebral angiography: a rare complication of the Angio-Seal arteriotomy closure device. J Cerebrovasc Endovasc Neurosurg 2014;16(3):275–280 Klocker J, Gratl A, Chemelli A, Moes N, Goebel G, Fraedrich G. Influence of use of a vascular closure device on incidence and surgical management of access site complications after percutaneous interventions. Eur J Vasc Endovasc Surg 2011;42(2):230–235 Wille J, Vos JA, Overtoom TT, Suttorp MJ, Van de Pavoordt ED, De Vries JP. Acute leg ischemia: the dark side of a percutaneous femoral artery closure device. Ann Vasc Surg 2006;20(2):278–281 A 70-year-old woman underwent coronary angiography via retrograde access to the common femoral artery (CFA). After successful treatment, a vascular closure device was used to achieve immediate hemostasis of the puncture site. One week later, the patient was admitted to the hospital with claudication (limited walking capacity 20 m). Before coronary angiography, no history of claudication was reported. Clinical examination at rest showed a pale ispsilateral forefoot (Fig. 4.84). Uneventful coronary angiography with application of a 6F ExoSeal vascular closure device (Cordis/Johnson & Johnson). The closure device was used according to instructions for use. However, after activation and sheath/ExoSeal removal, no hemostasis was achieved. Manual compression was needed for 5 minutes until complete hemostasis was achieved. Color duplex ultrasound of the groin and femoropopliteal artery. Embolization of the collagen plug into the distal femoral arterial lumen, resulting in severe obstruction (Fig. 4.85). • Endovascular management with stent placement (con: high material burden, femoral and popliteal flexion zone, near knee joint). • Ipsilateral antegrade puncture in order to extract the dislodged collagen by endovascular means such as snaring or aspiration. • Open surgical groin access with removal of foreign body and reconstruction of the CFA. This complication was solved by endovascular means and stent fixation of the collagen plug. An attempt to snare or aspirate the plug was unsuccessful. Only small amounts of the collagen material were removed with a snare (Figs. 4.86 and 4.87). An attempt at engaging the material with a stent retriever, originally intended for thrombus removal during acute stroke management, failed. The procedure was therefore completed by fixation of the collagen to the vessel wall using a self-expanding stent (SES) (5 × 40 mm) and final percutaneous transluminal angioplasty (PTA) (4 × 30 mm; Figs. 4.88 and 4.89). Finally, no signs of distal embolization at the origin of the below the knee arteries were visible (Fig. 4.90) and the foot arteries are patent. The main feeding vessels are the posterior and anterior tibial arteries (Fig. 4.91). Fig. 4.85 B-mode ultrasound showing the collagen plug (arrows) inside the vessel lumen adherent to an intravascular plaque. Fig. 4.87 Control angiography after several snaring attempts still shows a high-grade stenosis due to underling atherosclerotic plaque and dislodged collagen. Fig. 4.89 Angiography during 90° knee bending shows an uncompromised femoropopliteal artery segment. The reason for this complication remains unclear. During ExoSeal placement and activation it is probable that there was misplacement of the plug into the vessel lumen. • Evaluate the level of vessel access carefully in order to guarantee suitability for a vascular closure device (nondiseased, non–heavily calcified CFA, no deep femoral or superficial femoral artery puncture). • When experiencing unexpected friction or other irregularities while applying a closure device consider aborting the procedure and doing manual compression instead. • Follow the instructions for use carefully and do not hesitate to ask a colleague if something is unclear. Fig. 4.91 Even the foot arteries are patent. The main feeder vessels are the posterior and anterior tibial artery. Boersma D, Van Strijen MJ, Kloppenburg GT, Van den Heuvel DA, De Vries JP. Endovascular retrieval of a dislodged femoral arterial closure device with alligator forceps. J Vasc Surg 2012;55(4):1150–1152 Cahill TJ, Choji K, Kardos A. Fluoroscopy-guided snare retrieval of the celt ACD® metallic vascular closure device following failed deployment. Catheter Cardiovasc Interv 2014;83(4):556–559 Cikirikcioglu M, Cherian S, Keil V, et al. Surgical treatment of complications associated with the Angio-Seal vascular closure device. Ann Vasc Surg 2011;25(4):557.e1–e4 Kara K, Mahabadi AA, Rothe H, et al. Safety and effectiveness of a novel vascular closure device: a prospective study of the ExoSeal compared to the Angio-Seal and ProGlide. J Endovasc Ther 2014;21(6):822–828 Maxien D, Behrends B, Eberhardt KM, et al. Endovascular treatment of acute limb ischemia caused by an intravascularly deployed bioabsorbable plug of a vascular closure device. Vasa 2013;42(2):144–148 Schiele TM, Rademacher A, Meissner O, Klauss V, Hoffmann U. Acute limb ischemia after femoral arterial closure with a vascular sealing device: successful endovascular treatment. Vasa 2004;33(4):252–256 Suri S, Nagarsheth KH, Goraya S, Singh K. A novel technique to retrieve a maldeployed vascular closure device. J Endovasc Ther 2015;22(1):71–73 A 73-year-old woman suffering from breast cancer had received a port line 4 weeks previously. During routine lung X-ray, dislocation of the distal intravenous line into the right atrium was noticed; parts of the line protruded into the right ventricle. She had no clinical symptoms from this catheter dislodgement. A port line from the left subclavian vein (LSV) was placed 4 weeks ago. Placements of port line and port chamber were uneventful. During routine X-ray of the chest, a dislocation of the port catheter was noticed. Fluoroscopy of the chest. Fig. 4.92 Chest X-ray presenting a dislocation of the entire intravenous part of the port catheter into the right heart (atrium and partially ventricle). Port chamber and extravascular part of the port line are still in place. Posterior-anterior projection (a) and lateral projection (b). Port line dislocation into the right heart (Fig. 4.92a, b). • Foreign body removal under fluoroscopy. Snaring techniques from retrograde venous groin approach via a 12F sheath. A brachial or jugular approach should be avoided due to large sheath size. Puncture of the right common femoral vein (CFV) under local anesthesia and insertion of a 12F sheath. Advancement of a snare (different manufacturers available) and engagement of the port catheter. In this case, prior to the successful snaring maneuver, the port line was retracted and removed into the inferior vena cava (IVC) using a 5F Simmons catheter (Fig. 4.93). In the smaller IVC, snaring was successful and both snare and catheter were removed via the sheath (Figs. 4.94–4.96). After sheath removal, the procedure was completed by manual compression. Control fluoroscopy of the chest showed the extravenous part of the port line (Fig. 4.97). Disruption of the port line at the entrance level of the LSV. Decreased fatigue resistance of the catheter material resulted in catheter rupture and dislodgement into the right heart. • Inspect the port line for any material damage or fatigue prior to implantation. Fig. 4.94 Already snared port catheter from the distal end, which protruded into the IVC. The snare fixed the catheter in the mid-section. • Avoid any kinking or sharp manipulation of the port line. • Rule out any narrowing of the port line at the entrance level of the vessel. • Be careful with any sutures affecting the port line. Fig. 4.95 During the retrieval maneuver, the snare was repositioned carefully onto one of the port catheter ends in order to retrieve an unkinked catheter into the large sheath. Fig. 4.97 Final fluoroscopic control in order to rule out any further port catheter dislocation or disintegration after retrieval. The extravascular part of the port line was still in place. Bostan M, Durakoğlugil ME, Satiroğlu O, Erdivanli B, Tufan G. Retrieval of embolized tip of port catheter from branch of right pulmonary artery using a macro snare catheter. Interv Med Appl Sci 2014;6(2):93–95 Choksy P, Zaidi SS, Kapoor D. Removal of intracardiac fractured port-A catheter utilizing an existing forearm peripheral intravenous access site in the cath lab. J Invasive Cardiol 2014;26(2):75–76 Chuang MT, Wu DK, Chang CA, et al. Concurrent use of pigtail and loop snare catheters for percutaneous retrieval of dislodged central venous port catheter. Kaohsiung J Med Sci 2011;27(11):514–519 Colón-Casasnovas NE, Lugo-Vicente H. Distal fragmented port catheter: case report and review of literature. Bol Asoc Med P R 2008;100(1):70–75 Elkhoury MI, Boeckx WD, Chahine EG, Feghali MA. Retrieval of port-A catheter fragment from the main and right pulmonary arteries 3 years after dislodgement. J Vasc Access 2008;9(4):296–298 Hara M, Takayama S, Imafuji H, Sato M, Funahashi H, Takeyama H. Single-port retrieval of peritoneal foreign body using SILS port: report of a case. Surg Laparosc Endosc Percutan Tech 2011;21(3):e126–e129 Kawata M, Ozawa K, Matsuura T, et al. Percutaneous interventional techniques to remove embolized silicone port catheters from heart and great vessels. Cardiovasc Interv Ther 2012;27(3):196–200 Motta Leal Filho JM, Carnevale FC, Nasser F, et al. Endovascular techniques and procedures, methods for removal of intravascular foreign bodies. Rev Bras Cir Cardiovasc 2010;25(2):202–208 Sowinski H, Kobayashi D, Turner DR. Transcutaneous removal of an intravenous catheter fragment using a spider FX™ Embolic Protection device. Catheter Cardiovasc Interv 2015;86(3):467–471 Wang PC, Liang HL, Wu TH, et al. Percutaneous retrieval of dislodged central venous port catheter: experience of 25 patients in a single institute. Acta Radiol 2009;50(1):15–20 Wang SC, Tsai CH, Hou CP, et al. Dislodgement of port-A catheters in pediatric oncology patients: 11 years of experience. World J Surg Oncol 2013;11:191 A 74-year-old woman suffering from malignant lymphoma was scheduled for a port implantation before initiation of chemotherapy. During implantation, dislocation of entire port line occurred. Initial attempts to remove the catheter failed. Immediate chest X-ray showed the port catheter projecting into the right pulmonary artery trunk. Port line insertion from the left subclavian vein. During placement of the port line in the operating room, the entire catheter dislodged. Postoperative X-ray of the chest showed dislocation of the port catheter into the right pulmonary artery. Fluoroscopy of the chest. Port line dislocation into the right pulmonary artery (Fig. 4.98). • Foreign body removal under fluoroscopy. Snaring techniques from retrograde venous groin approach via a 12F sheath. A brachial or jugular approach should be avoided due to large sheath size. Puncture of the right common femoral vein (CFV) under local anesthesia and insertion of a 12F sheath. After placement of a 5F pigtail catheter into the right pulmonary artery a rotating maneuver was performed to engage the port catheter; however, it was unsuccessful. A 7F 90-cm-long sheath (Terumo) was therefore positioned into the right lower lobe artery. A 0.035-inch Teflon-coated wire was formed into a snare and advanced through the 7F sheath. Using the distal loop of the wire as a snare the port catheter was pulled back and fixed to the distal end of the sheath. During retrieval towards the main pulmonary artery, the port catheter was close to becoming dislodged again so it was held in that position. Then a regular 0.035-inch snare was advanced through the 7F sheath inside the 12F sheath. Passing the distal end of the long 7F sheath, the snare was used to grab the port catheter and finally remove it through the right heart into the inferior vena cava (IVC) (Figs. 4.99 and 4.100). Close to the groin, at the entrance level to the vein, both the snared catheter and the 12F sheath were fixed with a clamp because it was not possible to remove the entangled port catheter percutaneously (Fig. 4.101). Fig. 4.99 Fluoroscopy of the port catheter that has been snared and is ready for retraction into the IVC. Fig. 4.100 The entangled port catheter reached the distal part of the 12F sheath. Nevertheless, removal through the sheath failed. All material was removed surgically through veinotomy. Control fluoroscopy of the chest showed no remaining parts of the port line. During surgery the entire port line was lost through the entrance level of the left subclavian vein. Foreign body removal was begun endovascularly, but had to be completed surgically. • Be very aware of the port line at all times during implantation. • When preparing the port chamber, fixate the proximal end of the port line. Bostan M, Durakoğlugil ME, Satiroğlu O, Erdivanli B, Tufan G. Retrieval of embolized tip of port catheter from branch of right pulmonary artery using a macro snare catheter. Interv Med Appl Sci 2014;6(2):93–95 Choksy P, Zaidi SS, Kapoor D. Removal of intracardiac fractured port-A catheter utilizing an existing forearm peripheral intravenous access site in the cath lab. J Invasive Cardiol 2014;26(2):75–76 Chuang MT, Wu DK, Chang CA, et al. Concurrent use of pigtail and loop snare catheters for percutaneous retrieval of dislodged central venous port catheter. Kaohsiung J Med Sci 2011;27(11):514–519 Colón-Casasnovas NE, Lugo-Vicente H. Distal fragmented port catheter: case report and review of literature. Bol Asoc Med P R 2008;100(1):70–75 Elkhoury MI, Boeckx WD, Chahine EG, Feghali MA. Retrieval of port-A catheter fragment from the main and right pulmonary arteries 3 years after dislodgement. J Vasc Access 2008;9(4):296–298 Hara M, Takayama S, Imafuji H, Sato M, Funahashi H, Takeyama H. Single-port retrieval of peritoneal foreign body using SILS port: report of a case. Surg Laparosc Endosc Percutan Tech 2011;21(3):e126–3129. Kawata M, Ozawa K, Matsuura T, et al. Percutaneous interventional techniques to remove embolized silicone port catheters from heart and great vessels. Cardiovasc Interv Ther 2012;27(3):196–200 Motta Leal Filho JM, Carnevale FC, Nasser F, et al. Endovascular techniques and procedures, methods for removal of intravascular foreign bodies. Rev Bras Cir Cardiovasc 2010;25(2):202–208 Sowinski H, Kobayashi D, Turner DR. Transcutaneous removal of an intravenous catheter fragment using a spider FX™ Embolic Protection device. Catheter Cardiovasc Interv 2015;86(3):467–471 Wang PC, Liang HL, Wu TH, et al. Percutaneous retrieval of dislodged central venous port catheter: experience of 25 patients in a single institute. Acta Radiol 2009;50(1):15–20 Wang SC, Tsai CH, Hou CP, et al. Dislodgement of port-A catheters in pediatric oncology patients: 11 years of experience. World J Surg Oncol 2013;11:191 A 49-year-old woman with a history of acute lymphoblastic leukemia relapse was referred for central venous catheter placement. The patient had a platelet count of less than 15,000 and normal clotting parameters. A 6F double lumen peripherally inserted central catheter (PICC) was chosen for insertion through a standard arm approach. After repeatedly failed attempts to push the microintroducer’s guide-wire into a suitable arm vein, due to thrombotic occlusion, the peripheral access sites were abandoned. It was decided to place the line through a right subclavian vein (SCV) access. A sonographically guided puncture of the right SCV was made via an out-of-plane approach (transversal plane). The needle punctured the vein through-and-through with the first attempt, but after withdrawing the needle the guidewire could easily pass intraluminally and the line placement was completed as usual. Immediately after the puncture the patient experienced a paroxysmal cough and gross hemoptysis. Due to continuing hemoptysis on the ward she received a platelet transfusion, with no improvement. Fig. 4.102 CT ‘scout’ film showing central line placement through the right SCV. Note the steep angle of approach, due to out-of-plane ultrasound guidance, and also the right upper lobe density, immediately below the access site. The patient was referred for emergency chest computed tomography (CT) with an indication of life-threatening massive hemoptysis (Fig. 4.102). CT revealed a small peripheral pleural collection with contrast extravasation on the right upper lobe, just below the right SCV access site (Fig. 4.103). Lung window CT showed a large diffuse density in the right upper lobe, along with multiple focal densities in the middle and lower lung fields—findings consistent with diffuse pulmonary hemorrhage (Fig. 4.104). Due to continuing hemoptysis the patient developed dyspnea and tachypnea with resultant O2 saturation drop to around 60% and she was intubated. Based on contrast extravasation seen on CT scan the patient was referred for bronchial artery angiography and embolization. The angiography of subclavian artery, thyrocervical trunk, and bronchial arteries on the right was unremarkable (Fig. 4.105) and the patient was admitted to the Intensive Care Unit (ICU), transferred after 48 hours to the hematology ward, and discharged home a few days later. Fig. 4.103 Chest CT images showing a small contrast extravasation and pleural collection, bellow the SCV access site in the right upper lobe periphery (arrow). Fig. 4.105 Digital subtraction angiography of right thyrocervical trunk, subclavian artery, and bronchial arteries showing no contrast extravasation. Inadvertent through-and-through SCV puncture caused a small pleural and lung injury from the needle, initiating a paroxysmal cough reflex, which in turn, due to a low platelet count, caused massive pulmonary hemorrhage. • Ultrasound-guided SCV puncture via the out-of-plane approach (transversal plane) increases the risk of inadvertently puncturing the underlying pleura or lung, causing hemorrhage or pneumothorax. The sonographically guided in-plane approach (supra- or infraclavicular) is considered safer, because it allows for a better view of the needle tip. Additionally, an in-plane approach with a conventional linear probe forces the operator to use a more lateral access site, close to the axillary–subclavian vein junction where there is less risk of lung puncture. • A right internal jugular vein (IJV) approach would be a safer option, despite the inconvenience to the patient and possible increased infection rate. The patient was treated with intubation, platelet transfusion, and supportive therapy in the ICU. A small peripheral pleural lung extravasation on chest CT was not confirmed during angiography and no embolization was performed. Inadvertent pleura or lung puncture during right SCV catheterization in a patient with low platelets, causing paroxysmal cough and massive hemoptysis due to pulmonary hemorrhage in both lungs. • Patients with coagulation disorders are best treated by peripherally inserted lines, such as PICCs, but their prone-to-bleeding veins do not allow for multiple catheterization attempts. Adequate experience is essential. • A sonographically guided in-plane approach is a safer option for supra- or infraclavicular puncture of the SCV. • IJV is also a justified approach, despite inconvenience to the patient and possible increased infection rate. • Paroxysmal coughing can lead to serious complications in patients with severe coagulation disorders. Lennon M, Zaw NN, Pöpping DM, Wenk M. Procedural complications of central venous catheter insertion. Minerva Anestesiol 2012;78(11):1234–1240 Ruesch S, Walder B, Tramèr MR. Complications of central venous catheters: internal jugular versus subclavian access—a systematic review. Crit Care Med 2002; 30(2):454–460 A 46-year-old woman on hemodialysis (HD) presented with thrombosis of her arm HD fistula. It was decided to temporarily place a percutaneous dialysis catheter through the left common femoral vein (CFV). Percutaneous insertion of the temporary dialysis catheter into the left CFV using the traditional “blind” landmark method. During attempts to gain percutaneous access to the left CFV the patient developed a rapidly expanding upper thigh and labial hematoma (Fig. 4.106) and quickly became hemodynamically unstable. Fig. 4.106 Expanding left upper thigh and labial hematoma developed during attempts to gain percutaneous CFV access. Fig. 4.107 DSA through contralateral femoral approach demonstrates active contrast extravasation from left SEPA. Digital subtraction angiography (DSA) through percutaneous contralateral retrograde access to the right common femoral artery (CFA). Left superficial external pudendal artery (SEPA) injury with active bleeding (Figs. 4.107 and 4.108). Fig. 4.108 Selective left common femoral angiogram shows active contrast extravasation from a branch of SEPA (arrow) in more detail. • Catheterization of the feeder vessel and selective coil embolization. • Alternative agents for embolization: glue, large particles, gelfoam. • Stent graft implantation in the CFA in the unlikely occasion of failure to catheterize the SEPA. • Surgery (if endovascular approach fails). Endovascular treatment via contralateral retrograde CFA access was decided upon. The left SEPA was selectively catheterized with a 4F cobra catheter and successfully embolized in its proximal part with 4-mm platinum coils (Fig. 4.109). No attempt was made for a more distal superselective embolization due to the patient’s hemodynamic instability. Perforation of left SEPA branch during percutaneous CFV access attempts using the traditional “blind” landmark method. In general this complication is very rare during this type of procedure. This complication was managed by endovascular means. • Using ultrasound guidance instead of the traditional “blind” landmark technique for percutaneous femoral venous access is strongly advised. Fig. 4.109 Final angiogram demonstrating no further active extravasation due to successful coiling of the left proximal SEPA (arrow). • The use of ultrasound guidance is associated with significantly less risk of arterial puncture and hematomas and less time to insert venous catheters or sheaths, as well as a higher success rate for inserting the catheter or sheath on the first attempt. • Inadvertent arterial puncture during percutaneous femoral venous access can be life threatening, requiring immediate endovascular repair. • Interventionists performing CFV punctures should be familiar with the anatomy of the superficial and deep external pudendal arteries and be aware that these arteries could potentially be injured during the procedure. Baum PA, Matsumoto AH, Teitelbaum GP, Zuurbier RA, Barth KH. Anatomical relationship between the common femoral artery and vein: CT evaluation and clinical significance. Radiology 1989;173(3):775–777 A 77-year-old woman with a totally implantable vascular access device (TIVAD) for chemotherapy was referred to the surgeon who had initially performed the implantation for TIVAD removal. After the surgeon exposed the implanted port using the initial incision he noticed that the device was detached from its catheter. In an effort to secure the catheter in the subcutaneous tunnel a fairly large chest incision was made, but eventually the catheter was lost into the venous system. The patient was referred to the Interventional Radiology Unit (IRU) for percutaneous removal of the migrated TIVAD catheter. The catheter appeared in one piece on the chest X-ray (Fig. 4.110) with its central tip resting in the right ventricle and the peripheral tip in the right internal jugular vein (IJV). The TIVAD catheter was presumably cut during port exposure and during the attempt to grasp it the surgeon pushed it even further into the subcutaneous tissues and then into the venous system. • Accessing the venous system using a large sheath from the jugular or femoral approach, or both, in difficult cases, to create support. • Using a commercially available snare or making an improvised snare using a guide catheter or long sheath and a folded guidewire. • Refer the patient to a vascular surgeon to expose the peripheral tip of the catheter in the right IJV. The right common femoral vein (CFV) was accessed under local anesthesia and a short 10F vascular sheath was placed. A commercially available snare (SeQure 20-mm, Lifetech Scientific) was used to securely grasp the peripheral tip of the catheter, and the snare–catheter assembly was dragged into the 10F sheath and out of the body (Fig. 4.111a, b). The sheath was then removed and compression hemostasis applied. The patient was anticoagulated using a prophylactic dose of low molecular weight heparin (LMWH) to prevent pericatheter thrombosis and in the unlikely event of pulmonary embolism. During the immediate postoperative follow-up the patient complained of an enlarging painful hematoma around her chest incision. Visual inspection confirmed the increased swelling and she was referred for an emergency chest computed tomography (CT), which confirmed a subcutaneous hematoma presenting acute contrast extravasation from venous collaterals (Fig. 4.112). A vascular surgeon surgically drained the hematoma. Fig. 4.111 (a) Catheter fragment, step-by-step snaring, and removal through the right CFV sheath. (b) The snared catheter after removal. Accidental catheter cut during TIVAD removal is a common complication. Another possible cause may be the “pinch-off” phenomenon, where the catheter is chronically compressed and ruptured between the clavicle and the first rib. Pinch-off is rare nowadays due to the wide use of the IJV for central venous access. Fig. 4.112 Contrast-enhanced chest CT, showing a large subcutaneous hematoma formation (arrow) with acute contrast extravasation, caused by surgical exploration in an attempt to find the migrated catheter. • Some surgeons, for fear of catheter disconnection, prefer to use a separate incision from below to expose and remove a TIVAD. This leaves the patient with an extra scar and is rarely, if ever, necessary if some simple rules are followed. • Familiarity with the TIVAD that needs removal is crucial. To assess the type of device implanted, study the patient’s files and if no information can be found perform a preoperative chest radiograph. Most devices have a unique appearance on X-rays. • A chest radiograph is obviously indicated when looking for a detached catheter, to determine its location and whether it is in one piece—factors that can affect the removal plan. • When exposing a TIVAD, blunt dissection using scissors or a simple mosquito forceps is preferable because catheters are notoriously easy to cut! Moreover, some devices have a small plastic safety sleeve to connect the port to the catheter, which can be easily removed if force is applied. • To implant TIVADs, sonographically guided IJV punctures are preferable, which, among other benefits, will prevent “pinch-off” syndrome. Moreover, in the case of accidental catheter dislodgement during removal, the catheter tip is almost always lost in subcutaneous tissues and every attempt to grasp it pushes it even deeper. It is hopeless to chase a migrated catheter tip with forceps. Instead, the catheter may be easily exposed and removed from the IJV puncture. Bostan M, Durakoğlugil ME, Satiroğlu O, Erdivanli B, Tufan G. Retrieval of embolized tip of port catheter from branch of right pulmonary artery using a macro snare catheter. Interv Med Appl Sci 2014;6(2):93–95 Choksy P, Zaidi SS, Kapoor D. Removal of intracardiac fractured port-A catheter utilizing an existing forearm peripheral intravenous access site in the cath lab. J Invasive Cardiol 2014;26(2):75–76 Chuang MT, Wu DK, Chang CA, et al. Concurrent use of pigtail and loop snare catheters for percutaneous retrieval of dislodged central venous port catheter. Kaohsiung J Med Sci 2011;27(11):514–519 Colón-Casasnovas NE, Lugo-Vicente H. Distal fragmented port catheter: case report and review of literature. Bol Asoc Med P R 2008;100(1):70–75 Elkhoury MI, Boeckx WD, Chahine EG, Feghali MA. Retrieval of port-A catheter fragment from the main and right pulmonary arteries 3 years after dislodgement. J Vasc Access 2008;9(4):296–298 Hara M, Takayama S, Imafuji H, Sato M, Funahashi H, Takeyama H. Single-port retrieval of peritoneal foreign body using SILS port: report of a case. Surg Laparosc Endosc Percutan Tech 2011;21(3):e126–e129 Kawata M, Ozawa K, Matsuura T, et al. Percutaneous interventional techniques to remove embolized silicone port catheters from heart and great vessels. Cardiovasc Interv Ther 2012;27(3):196–200 Motta Leal Filho JM, Carnevale FC, Nasser F, et al. Endovascular techniques and procedures, methods for removal of intravascular foreign bodies. Rev Bras Cir Cardiovasc 2010;25(2):202–208 Sowinski H, Kobayashi D, Turner DR. Transcutaneous removal of an intravenous catheter fragment using a spider FX™ Embolic Protection device. Catheter Cardiovasc Interv 2015;86(3):467–471 Wang PC, Liang HL, Wu TH, et al. Percutaneous retrieval of dislodged central venous port catheter: experience of 25 patients in a single institute. Acta Radiol 2009;50(1):15–20 Wang SC, Tsai CH, Hou CP, et al. Dislodgement of port-A catheters in pediatric oncology patients: 11 years of experience. World J Surg Oncol 2013;11:191 A 78-year-old woman was scheduled for central venous line placement to facilitate hemofiltration therapy on the Intensive Care Unit (ICU) after surgery. Right-side subclavian catheter placement (10F). Pulsatile arterial backflow during incidental placement of the venous line into the subclavian artery. Angiography via the catheter in order to verify the intra-arterial position and to determine the arterial puncture level. Inadvertent subclavian arterial puncture and large lumen catheter placement (Figs. 4.113 and 4.114). • Insertion of a 0.035-inch guidewire and vascular closure with an 8F Angio-Seal (e.g., St. Jude Medical). • Insertion of a 0.035-inch guidewire and vascular closure with one or two suture-mediated closure devices (e.g., Abbott Vascular 6F ProGlide or 10F Prostar). • Transarterial balloon tamponade. • Implantation of a stent graft after removal of the arterial “central line.” • Manual compression of the subclavian puncture site (unlikely to be successful). • Surgery (if an endovascular approach fails). Retrograde puncture of the right groin and 7F sheath insertion. Diagnostic angiography for anatomical evaluation of the aortic arch. Catheterization of the right subclavian artery and prophylactic positioning of an 8 × 40-mm balloon for short-term inflation during the catheter retrieval. Fig. 4.114 Angiography via a 4F pigtail catheter placed in the aortic arch. Note the correct position of the contralateral left-side central line ending in the proximal superior vena cava. Fig. 4.115 Catheterization of the right-side subclavian artery and positioning of an 8 × 40-mm balloon for short-term inflation during catheter retrieval and sheath insertion for the Angio-Seal closure device. After placement of a 0.035-inch guidewire through the central line, and removal of the catheter, an Angio-Seal closure device was introduced (Figs. 4.115 and 4.116). “Off-label” application of the 8F Angio-Seal device. Unfortunately, control angiography of the left subclavian artery (LSA) showed contrast extravasation at the puncture site level (Fig. 4.117). The decision was made to implant a covered stent from the groin (Viabahn 8 × 50 mm). After stent graft placement, the subclavian artery was sealed (Fig. 4.118). Fig. 4.116 Placement of an 8F Angio-Seal closure device, while the balloon catheter is inflated in order to avoid any bleeding during device exchange and positioning. Puncture of the subclavian vein without ultrasound guidance resulted in inadvertent subclavian artery puncture and large-bore arterial central line insertion. • When subclavian vein puncture for central line placement is difficult, a B-mode ultrasound should be used in order to gather further anatomical information. • Once a subclavian vessel has been punctured, the venous position should be verified before a large-bore catheter is inserted. • If there is arterial backflow from the needle it should be removed and manual compression performed. An increase of the access diameter by inserting the catheter should be avoided. • Perform every vessel puncture with great care even if the procedure seems simple! • Use B-mode ultrasound, whenever possible. Chan CC, Lee V, Chu W, Tam YH, Li CK, Shing MM. Carotid jugular arteriovenous fistula: an unusual complication of internal jugular vein catheterization in children. Pediatr Blood Cancer 2012;59(7):1302–1304 Lennon M, Zaw NN, Pöpping DM, Wenk M. Procedural complications of central venous catheter insertion. Minerva Anestesiol 2012;78(11):1234–1240 Ruesch S, Walder B, Tramèr MR. Complications of central venous catheters: internal jugular versus subclavian access—a systematic review. Crit Care Med 2002;30(2):454–460 Schütz N, Doll S, Bonvini RF. Erroneous placement of central venous catheter in the subclavian artery: retrieval and successful hemostasis with a femoral closure device. Catheter Cardiovasc Interv 2011;77(1):154–157 A 54-year-old woman came to the outpatient oncology clinic for a chemotherapy follow-up appointment for breast cancer. Attempts to flush the Port-A-Cath device were unsuccessful. A chest X-ray was performed. Four years previously the patient had been diagnosed with breast cancer. As part of the treatment regimen a Port-A-Cath device was inserted for central venous access for chemotherapy. There were no problems encountered during the initial insertion of the Port-A-Cath device based on a review of the procedure notes and postprocedure imaging from 4 years prior. Usually, no dedicated additional imaging is required, because the dislocated catheter is already visualized by chest X-ray. If this was not yet done, a chest X-ray is indicated for exact catheter location. Chest X-ray performed at time of current presentation demonstrated separation of the catheter from the central venous port hub (Fig. 4.119). • Endovascular treatment via right internal jugular vein to snare catheter fragment. • Endovascular treatment via right common femoral vein (CFV) to snare catheter fragment. Endovascular access was obtained via the right CFV under ultrasound guidance using a micropuncture kit. An 8F vascular sheath was inserted. Using a 0.035-inch 145-cm-long Rosen wire and an 80-cm-long angled 5F MPA (multipurpose A) catheter, cannulation into the inferior vena cava (IVC) was performed. Fluoroscopy showed the catheter fragment overlying the upper chest. The angled MPA catheter and wire were advanced into the superior vena cava (SVC). Contrast was injected through the MPA catheter and a central venogram was done (Fig. 4.120). Venogram showed the proximal tip of the catheter located in the right brachiocephalic vein and the distal tip in the right ventricle based on catheter position. Neither the proximal nor the distal tip could be grabbed with a loop snare as the proximal tip was embedded in the vein wall and the distal tip appeared to be located near the tricuspid valve. Using a reverse curve VS-1 catheter, the middle of the catheter was hooked. The reverse-curve catheter was twisted and the catheter fragment brought down into the suprarenal IVC. At this point a 20-mm loop snare was used to engage the proximal edge of the catheter. The snare was brought to the middle of the catheter fragment, tightened, and the catheter was cinched down (Fig. 4.121). The catheter fragment was then infolded and delivered through an 8F sheath from the right CFV access (Fig. 4.122). Fig. 4.120 Venogram showing the proximal tip of the catheter located in the right brachiocephalic vein and the distal tip in the right ventricle based on catheter position. Fluoroscopy of the chest and abdomen demonstrated no evidence of any retained catheter fragments (Fig. 4.123). Using local anesthetic and blunt dissection, the port device was removed from the right anterior chest. Fracture, dehiscence, and separation of central venous access devices are not uncommon. Removing endovascular foreign bodies is one of the key skills of an interventional radiologist. In this case neither the proximal nor the distal tip of the catheter could be snared in the chest, which is not uncommon. “Hooking” the catheter fragment using a reverse-curve catheter is a useful technique to expose the edges of the catheter for a snaring maneuver in the IVC in a “lower risk environment.” • Intravascular foreign bodies are a common occurrence. Having multiple techniques and access points for retrieval is critical. • Carefully delineating the anatomy with endovascular contrast is critical prior to any intervention. • Always have the equipment and a plan in place if your initial access site or equipment is unsuccessful in retrieving the intravascular foreign body. Bostan M, Durakoğlugil ME, Satiroğlu O, Erdivanli B, Tufan G. Retrieval of embolized tip of port catheter from branch of right pulmonary artery using a macro snare catheter. Interv Med Appl Sci 2014;6(2):93–95 Choksy P, Zaidi SS, Kapoor D. Removal of intracardiac fractured port-A catheter utilizing an existing forearm peripheral intravenous access site in the cath lab. J Invasive Cardiol 2014;26(2):75–76 Chuang MT, Wu DK, Chang CA, et al. Concurrent use of pigtail and loop snare catheters for percutaneous retrieval of dislodged central venous port catheter. Kaohsiung J Med Sci 2011;27(11):514–519 Colón-Casasnovas NE, Lugo-Vicente H. Distal fragmented port catheter: case report and review of literature. Bol Asoc Med P R 2008;100(1):70–75 Elkhoury MI, Boeckx WD, Chahine EG, Feghali MA. Retrieval of port-A catheter fragment from the main and right pulmonary arteries 3 years after dislodgement. J Vasc Access 2008;9(4):296–298 Hara M, Takayama S, Imafuji H, Sato M, Funahashi H, Takeyama H. Single-port retrieval of peritoneal foreign body using SILS port: report of a case. Surg Laparosc Endosc Percutan Tech 2011;21(3):e126–e129 Kawata M, Ozawa K, Matsuura T, et al. Percutaneous interventional techniques to remove embolized silicone port catheters from heart and great vessels. Cardiovasc Interv Ther 2012;27(3):196–200 Motta Leal Filho JM, Carnevale FC, Nasser F, et al. Endovascular techniques and procedures, methods for removal of intravascular foreign bodies. Rev Bras Cir Cardiovasc 2010;25(2):202–208 Sowinski H, Kobayashi D, Turner DR. Transcutaneous removal of an intravenous catheter fragment using a spider FX™ Embolic Protection device. Catheter Cardiovasc Interv 2015;86(3):467–471 Wang PC, Liang HL, Wu TH, et al. Percutaneous retrieval of dislodged central venous port catheter: experience of 25 patients in a single institute. Acta Radiol 2009;50(1):15–20 Wang SC, Tsai CH, Hou CP, et al. Dislodgement of port-A catheters in pediatric oncology patients: 11 years of experience. World J Surg Oncol 2013;11:191 An obese, elderly woman presented with problems with her permacath. Dialysis nurses reported that injection inflow was possible but aspiration was limited. Thrombosis of one of the catheter channels was suspected. A standard in the dialysis practice of one of the contributing authors is to inject 10 mg of recombinant tissue plasminogen activator (rtPA) in 1.9 mL normal saline (the dead space volume of the catheter) into each lumen. This avoids any systemic thrombolysis. When this failed to improve blood flow the patient was referred to angiography. Angiography revealed an extensive fibrin sheath with a large thrombus at the distal end of the permacath projecting into the right atrium (Fig. 4.124). Anticoagulation was begun with low molecular weight heparin (LMWH) (enoxaparin). During flushing of the permacath for angiography, the catheter began to extrude until the fibrin cuff, previously in a subcutaneous tunnel, appeared at the skin surface. Computed tomography (CT) venogram and further angiography. Imaging revealed a large atrial thrombus and extensive fibrin sheath with partial occlusion of the superior vena cava (SVC). The permacath had extruded further almost to the junction of the subclavian and jugular veins. The patient had been off dialysis for 3 days. Fig. 4.124 Angiography revealed an extensive fibrin sheath with a large thrombus at the distal end of the permacath projecting into the right atrium. • Insertion of alternative venous access: • Patient refused as she had had two prior failed permacaths via the left internal jugular vein. • Femoral vein access was considered risky because of obesity and infection risk. • Removal of the permacath and hope the thrombus does not detach from the catheter. • Insertion of a new permacath from higher in the right internal jugular vein or subclavian vein. • Open thoracotomy and thrombectomy. • Replacement of permacath with protection against pulmonary embolus. Via an ultrasound guided femoral vein puncture in the thigh, a long 12F venous sheath was inserted into the hepatic portion of the inferior vena cava (IVC). A 24-mm-diameter self-expanding sinus-XL nitinol stent (OptiMed) was partially deployed immediately below the atrial thrombus to act as a trap for any loose thrombus (Fig. 4.125). A 5F Kumpe catheter was then inserted into the right internal jugular vein and a wire guide passed beyond the thrombus. Since the thrombus deflected the wire guide either into the right atrium (Fig. 4.126) or into the open stent (Fig. 4.127) the stent was closed around the wire guide and withdrawn until the guidewire could be released in the IVC (Fig. 4.128). The nitinol stent was then repositioned partly opened below the thrombus and the permacath removed. Fig. 4.125 Angiography via diagnostic catheter showing a 24-mm diameter self-expanding Optimed Sinus XL nitinol stent, partially deployed immediately below the atrial thrombus to act as a trap for any loose thrombus. Fig. 4.127 The stent was closed around the wire guide and withdrawn until the guidewire could be released in the inferior vena cava. Fig. 4.129 A new long permacath inserted from the high right internal jugular puncture down to the IVC, flushed and heparin locked. A new long permacath was inserted from the high right internal jugular puncture down to the IVC, flushed and heparin locked (Fig. 4.129). Once it was apparent the thrombus was not mobile, the nitinol stent was sheathed and removed. Anticoagulation has been continued with the expectation that the thrombus will resolve completely. Inadequate flushing and failure to recognize a fibrin sheath early resulted in a potentially life-threatening thrombus formation. • Permacath withdrawal is more common in obese patients especially if the tunneled portion is long and in a pendulant portion of the chest. • Early management of fibrin sheaths is preferred. • Think outside the box for solutions. • Know how far a stent can be deployed before it cannot be recaptured. Gabriel J. Preventing and managing complications of CVADs. Nurs Times 2013;109(40):20–23 Gaddh M, Antun A, Yamada K, et al. Venous access catheter-related thrombosis in patients with cancer. Leuk Lymphoma 2014;55(3):501–508 Latham GJ, Thompson DR. Thrombotic complications in children from short-term percutaneous central venous catheters: what can we do? Paediatr Anaesth 2014;24(9):902–911 Mino JS, Gutnick JR, Monteiro R, Anzlovar N, Siperstein AE. Line-associated thrombosis as the major cause of hospital-acquired deep vein thromboses: an analysis from National Surgical Quality Improvement Program data and a call to reassess prophylaxis strategies. Am J Surg 2014;208(1):45–49 Nayeemuddin M, Pherwani AD, Asquith JR. Imaging and management of complications of central venous catheters. Clin Radiol 2013;68(5):529–544 Zochios V, Umar I, Simpson N, Jones N. Peripherally inserted central catheter (PICC)-related thrombosis in critically ill patients. J Vasc Access 2014;15(5):329–337 A 69-year-old patient suffered from acute abdominal and right flank pain, developing several hours following diagnostic coronary angiography via retrograde access to the right common femoral artery (CFA). Apart from his coronary heart disease no further comorbidities were reported. During coronary angiography a vascular closure device was used successfully and immediate hemostasis of the puncture site was achieved. The patient now presented with clinical signs of hypovolemia, controllable by means of fluid resuscitation. Clinical inspection of the right groin did not show significant hematoma or active bleeding. Diagnostic coronary angiography. Unremarkable procedure, no complications reported! Computed tomography angiography (CTA). There is active retroperitoneal bleeding with a huge right retroperitoneal hematoma proven by contrast-enhanced CTA (Figs. 4.130 and 4.131). • Endovascular treatment via ipsilateral or contralateral retrograde access to the CFA. Fig. 4.131 Coronal maximum intensity projection (MIP) reconstructions show the feeder vessel (deep circumflex iliac artery) and the location of active bleeding (arrow). • Catheterization of the feeder vessel, for example using a microcatheter and followed by selective microcoil embolization. • Alternative agents for embolization: glue, large particles, gelfoam. • Surgery (if an endovascular approach fails). Endovascular treatment via ipsilateral retrograde access to the CFA (4F sheath). Catheterization of the feeder vessel with a microcatheter (2.7F) followed by selective microcoil embolization (Figs. 4.132 and 4.133). Suspected uncontrolled advancement of a hydrophylic guidewire during the initial procedure (after further interviewing the operating cardiologist), resulted in side-branch perforation. • Guidewire advancement should be constantly controlled under fluoroscopy, especially when using hydrophilic-coated wires! • Wires should never be pushed against resistance without fluoroscopic guidance! Axelrod DJ, Freeman H, Pukin L, Guller J, Mitty HA. Guidewire perforation leading to fatal perirenal hemorrhage from transcortical collaterals after renal artery stent placement. J Vasc Interv Radiol 2004;15(9):985–987 Blake PG, Uldall R. Cardiac perforation by a guidewire during subclavian catheter insertion. Int J Artif Organs 1989;12(2):111–113 Durão C, Barros A, Guerreiro R, Pedrosa F. “Death by a thread”—peritonitis due to visceral perforation by a guidewire, during proximal femur osteosynthesis with DHS: a fatal case and legal implications. Forensic Sci Int 2015;249:e12–e14 Hiroshima Y, Tajima K, Shiono Y, et al. Soft J-tipped guide-wire-induced cardiac perforation in a patient with right ventricular lipomatosis and wall thinning. Intern Med 2012;51(18):2609–2612 Hong YM, Lee SR. A case of guidewire fracture with remnant filaments in the left anterior descending coronary artery and aorta. Korean Circ J 2010;40(9):475–477 Lee SY, Kim SM, Bae JW, et al. Renal artery perforation related with hydrophilic guidewire during coronary intervention: successful treatment with polyvinyl alcohol injection. Can J Cardiol 2012;28(5):612.e5–e7 Tanaka S, Nishigaki K, Ojio S, et al. Transcatheter embolization by autologous blood clot is useful management for small side-branch perforation due to percutaneous coronary intervention guidewire. J Cardiol 2008;52(3):285–289 Störger H, Ruef J. Closure of guidewire-induced coronary artery perforation with a two-component fibrin glue. Catheter Cardiovasc Interv 2007;70(2):237–240 A 61-year-old male patient suffering from peripheral artery occlusive disease (PAOD) presented a 10-cm occlusion of the superficial femoral artery (SFA) during magnetic resonance angiography (MRA). The patient was scheduled for SFA recanalization. After antegrade groin access and administration of 5,000 IU of heparin and 1,000 mg ASA (aspirin) intravenously, the lesion was recanalized successfully using a hydrophilic-coated 0.018-inch wire. The procedure was completed by placement of a self-expanding stent. During the procedure, the patient complained of a short, self-limiting, heavy pain localized in the ipsilateral calf. The operator instantly retracted the guidewire for 4–5 cm, with its tip being left distally from the treated lesion. Control angiography for run-off evaluation showed contrast extravasation of the proximal and distal inferior medial genicular artery. SFA recanalization via antegrade approach followed by plain old balloon angioplasty (POBA) and stent placement (self-expanding stent [SES]). Apart from the patient complaining of a short episode of heavy sharp pain in the calf during POBA and stent placement, the intervention was uneventful. However, bleeding from an infragenual side branch was noticed during control angiography (Fig. 4.134). Selective/superselective angiography in order to visualize the exact source of bleeding (Fig. 4.135). Side-branch perforation. • Manual compression of the calf at the level of the detected bleeding source for 3 minutes. Repetition of manual compression procedure until bleeding stops. • Placement of a pressure cuff, which is applied for 3 minutes between systolic and diastolic pressure; pedal pulses should be palpable; repetition for another 3 minutes if warranted; inflation for 1–3 minutes to 20 mm Hg suprasystolic pressure might be helpful in dedicated situations resistant to mild compression (adapted from the instructions for use for FemoStop [St. Jude Medical] compression clamp). Fig. 4.134 Digital subtraction angiography (DSA) in a lateral projection and 90° knee bending after stent placement in the mid-part SFA. A moderate contrast blush (arrow) was noticed at the level of the inferior medial genicular artery. • Selective catheterization of the feeder vessel using a microcatheter with embolization (coil, glue, collagen sponge). Fig. 4.136 Final angiographic control after 20 minutes of manual compression. No signs of residual bleeding were visible. Manual compression of the calf at the level of the detected bleeding source for 3 minutes. Repetition of the compression procedure until bleeding stopped. Final hemostasis was verified by control angiography (Fig. 4.136). Uncontrolled hydrophilic guidewire advancement resulted in perforation of a calf vessel side branch. • Always be aware of the position of the distal end of hydrophilic guidewires. • Secure wires carefully during catheter or device exchange (advancement and removal). • Whenever possible, keep visual control over the hydrophilic tip of the guidewire. • Take patients’ complaints seriously and check what could be responsible for the onset of new symptoms during the intervention. Axelrod DJ, Freeman H, Pukin L, Guller J, Mitty HA. Guidewire perforation leading to fatal perirenal hemorrhage from transcortical collaterals after renal artery stent placement. J Vasc Interv Radiol 2004;15(9):985–987 Blake PG, Uldall R. Cardiac perforation by a guidewire during subclavian catheter insertion. Int J Artif Organs 1989;12(2):111–113 Durão C, Barros A, Guerreiro R, Pedrosa F. “Death by a thread”—peritonitis due to visceral perforation by a guidewire, during proximal femur osteosynthesis with DHS: a fatal case and legal implications. Forensic Sci Int 2015;249:e12–e14 Hiroshima Y, Tajima K, Shiono Y, et al. Soft J-tipped guide-wire-induced cardiac perforation in a patient with right ventricular lipomatosis and wall thinning. Intern Med 2012;51(18):2609–2612 Hong YM, Lee SR. A case of guidewire fracture with remnant filaments in the left anterior descending coronary artery and aorta. Korean Circ J 2010;40(9):475–477 Lee SY, Kim SM, Bae JW, et al. Renal artery perforation related with hydrophilic guidewire during coronary intervention: successful treatment with polyvinyl alcohol injection. Can J Cardiol 2012;28(5):612.e5–e7 Störger H, Ruef J. Closure of guidewire-induced coronary artery perforation with a two-component fibrin glue. Catheter Cardiovasc Interv 2007;70(2):237–240 Tanaka S, Nishigaki K, Ojio S, et al. Transcatheter embolization by autologous blood clot is useful management for small side-branch perforation due to percutaneous coronary intervention guidewire. J Cardiol 2008;52(3):285–289 A 68-year-old man suffering from peripheral artery occlusive disease (PAOD) presented with multiple high-grade stenoses of the superficial femoral artery (SFA) during diagnostic magnetic resonance angiography (MRA). The patient was scheduled for SFA percutaneous transluminal angioplasty (PTA) and optional stenting. After antegrade groin access and administration of 5,000 IU of heparin and 1000 mg ASA (aspirin) intravenously, the lesions were passed successfully using a hydrophilic-coated 0.018-inch wire. The procedure was completed by placement of multiple self-expanding stents (SESs) due to multilevel flow-limiting dissections and elastic recoil despite repeated long-term plain old balloon angioplasty (POBA). Several devices (PTA catheters and stent delivery catheters) had been advanced, removed, and exchanged; a 0.018-inch guidewire was left in place during the entire procedure. During the procedure (probably during device removal or advancement), the patient complained of a short, self-limiting, heavy pain attack in the ipsilateral calf. The guidewire was retracted immediately for a few centimeters and positioned in the popliteal artery (PIII), still distal from the treated lesion. During control angiography, extravasation of contrast media in two different infragenual side branches was depicted (Fig. 4.137). SFA multilevel lesion treatment via an antegrade approach followed by POBA and stent placement (SESs). Fig. 4.137 Digital subtraction angiography showing a moderate contrast blush (arrows) at the level of the inferior medial genicular artery and more distally from a peroneal artery side branch. Apart from the patient complaining of a self-limiting severe sharp pain during POBA and stent placement, the intervention was uneventful. However, bleeding from infragenual side branches was noticed during control angiography. Selective angiography in order to visualize the exact source of bleeding. Side-branch perforation. • Manual compression of the calf at the level of detected bleeding source for 3 minutes. Repetition of compression procedure until bleeding stops. • Placement of a pressure cuff, which is applied for 3 minutes between systolic and diastolic pressure; pedal pulses should be palpable; repetition for another 3 minutes if warranted; short-term inflation for 1–3 minutes to 20 mm Hg suprasystolic pressure might be helpful in dedicated situations resistant to mild compression (adapted from the instructions for use for FemoStop [St. Jude Medical] compression clamp). • Selective catheterization of the feeder vessel by microcatheter and embolization (coil, glue, collagen sponge). Inflation of a pressure cuffplaced below the knee at the level of detected bleeding source for 3 minutes at diastolic pressure. Repetition of compression procedure by carefully inflating the cuff until bleeding stopped as proven in the final angiographic control (Fig. 4.138). Proof of hemostasis by control angiography. Clinical follow-up with inspection of the calf and Doppler assessment to rule out development of compartment syndrome. Uncontrolled hydrophilic guidewire advancement resulted in perforation of calf vessel side branches. Fig. 4.138 Angiography via 4F angle-tip diagnostic catheter and 0.018-inch guidewire in place. Significant contrast extravasation was no longer visible. • Always be aware of the position of the distal end of hydrophilic guidewires. • Secure wires carefully during catheter or device exchange (advancement and removal). • Whenever possible, keep visual control over the hydrophilic tip of the guidewire. • Take patients’ complaints seriously and check what could be responsible for the onset of new symptoms during the intervention. Axelrod DJ, Freeman H, Pukin L, Guller J, Mitty HA. Guidewire perforation leading to fatal perirenal hemorrhage from transcortical collaterals after renal artery stent placement. J Vasc Interv Radiol 2004;15(9):985–987 Blake PG, Uldall R. Cardiac perforation by a guidewire during subclavian catheter insertion. Int J Artif Organs 1989;12(2):111–113 Durão C, Barros A, Guerreiro R, Pedrosa F. “Death by a thread”—peritonitis due to visceral perforation by a guidewire, during proximal femur osteosynthesis with DHS: a fatal case and legal implications. Forensic Sci Int 2015;249:e12–e14 Hiroshima Y, Tajima K, Shiono Y, et al. Soft J-tipped guide-wire-induced cardiac perforation in a patient with right ventricular lipomatosis and wall thinning. Intern Med 2012;51(18):2609–2612 Hong YM, Lee SR. A case of guidewire fracture with remnant filaments in the left anterior descending coronary artery and aorta. Korean Circ J 2010;40(9):475–477 Lee MS, Applegate B, Rao SV, Kirtane AJ, Seto A, Stone GW. Minimizing femoral artery access complications during percutaneous coronary intervention: a comprehensive review. Catheter Cardiovasc Interv 2014;84(1):62–69 Störger H, Ruef J. Closure of guidewire-induced coronary artery perforation with a two-component fibrin glue. Catheter Cardiovasc Interv 2007;70(2):237–240 Tanaka S, Nishigaki K, Ojio S, et al. Transcatheter embolization by autologous blood clot is useful management for small side-branch perforation due to percutaneous coronary intervention guidewire. J Cardiol 2008;52(3):285–289 A 62-year-old patient suffered from a pulseless right brachial artery 4 weeks after percutaneous coronary intervention (PCI; drug-eluting stent [DES] left anterior descending [LAD]) via a transradial approach. Comorbidities were hypertension and smoking history for 10 years. Since discharge after PCI, the patient was on ASA (aspirin), statins, and antihypertensive triple therapy. Two weeks after PCI, the patient reported arm pain during stress and motoric weakness. A symptomatic distal subclavian or axillary artery stenosis was suspected based on clinical and Doppler ultrasound examination. PCI was planned using a right-side transradial approach. A guide catheter of unknown size (6F or 7F) failed to catheterize the left coronary artery. Attempts to exchange the catheter via a guidewire failed. A snaring maneuver from the groin, after completing PCI from an inguinal approach, was successful for catheter removal. Final angiography of the brachiocephalic trunk and the subclavian artery showed no damage of the vessels, where the catheter kinked (angiogram not available). Patient’s discharge was uneventful. Doppler ultrasound 3 days after PCI depicted triphasic flow signals for all peripheral right arm arteries (axillary, brachial, radial, and ulnar). Two weeks later the patient presented with symptoms, as described above. Failed placement of a guiding catheter via a transradial approach in order to perform PCI. Manipulation with the guiding catheter resulted in catheter kinking. Catheter removal via a radial approach failed. In order to avoid any vessel damage, the distal and kinked catheter was snared from a groin approach and successfully removed (no images available). Attempts made to overcome this problem using a hydrophilic-coated guidewire resulted in scaling of the coating. Angiography of the right upper extremity from the groin. Short-distance circumscriptive flow-limiting dissection of the right axillary artery (Fig. 4.139). • Endovascular treatment via retrograde groin access from a common femoral artery (CFA) or retrograde radial/brachial ipsilateral access. Fig. 4.139 Diagnostic, selective angiography of the right subclavian artery shows a short-distance, circumscriptive flow-limiting dissection of the right axillary artery. • Crossing the lesion and performing long-term percutaneous transluminal angioplasty (PTA) or cutting-balloon angioplasty (CBA), if PTA is not sufficient. • Surgery (if an endovascular approach fails). Endovascular treatment via retrograde groin access from the right CFA (6F sheath, 90 cm long). The sheath was placed in the subclavian artery. A 4F JB catheter (125 cm) was placed in front of the lesion. Wire (0.018-inch Terumo) crossing failed (Fig. 4.140). The reverse side of an 0.018-inch wire could pass the dissection membrane. After verified successful lesion crossing with the catheter, the reverse-side flexible wire tip was placed beyond the lesion and long-term PTA with a 7 × 40-mm balloon was performed (Fig. 4.141). Final angiography in neutral (Fig. 4.142) and elevated (Fig. 4.143) arm position showed an immediate contrast run-off; peripheral pulses were palpable. Fig. 4.141 After successful lesion crossing (with reverse side of the Terumo wire), long-term PTA with a 7 × 40-mm balloon was performed. A manipulation of a guide catheter placed for transradial PCI led to catheter kinking in the axillary artery, resulting in a flow-limiting dissection. • Careful manipulation of endovascular instruments. • If a severe resistance is noticed or unknown forces are acting during guidewire or (guide) catheter placement and manipulation, all further manipulations should be carried out with great care. • All wire and catheter advancements should be done under fluoroscopic control. Axelrod DJ, Freeman H, Pukin L, Guller J, Mitty HA. Guidewire perforation leading to fatal perirenal hemorrhage from transcortical collaterals after renal artery stent placement. J Vasc Interv Radiol 2004;15(9):985–987 Blake PG, Uldall R. Cardiac perforation by a guidewire during subclavian catheter insertion. Int J Artif Organs 1989;12(2):111–113 Durão C, Barros A, Guerreiro R, Pedrosa F. “Death by a thread”—peritonitis due to visceral perforation by a guidewire, during proximal femur osteosynthesis with DHS: a fatal case and legal implications. Forensic Sci Int 2015;249:e12–e14 Hiroshima Y, Tajima K, Shiono Y, et al. Soft J-tipped guide-wire-induced cardiac perforation in a patient with right ventricular lipomatosis and wall thinning. Intern Med 2012;51(18):2609–2612 Hong YM, Lee SR. A case of guidewire fracture with remnant filaments in the left anterior descending coronary artery and aorta. Korean Circ J 2010;40(9):475–477 Lee SY, Kim SM, Bae JW, et al. Renal artery perforation related with hydrophilic guidewire during coronary intervention: successful treatment with polyvinyl alcohol injection. Can J Cardiol 2012;28(5):612.e5–e7 Störger H, Ruef J. Closure of guidewire-induced coronary artery perforation with a two-component fibrin glue. Catheter Cardiovasc Interv 2007;70(2):237–240 Tanaka S, Nishigaki K, Ojio S, et al. Transcatheter embolization by autologous blood clot is useful management for small side-branch perforation due to percutaneous coronary intervention guidewire. J Cardiol 2008;52(3):285–289 A 55-year-old woman on chronic dialysis had a failed internal jugular vein (IJV) catheter due to superior vena cava (SVC) obstruction and subsequently a temporary femoral catheter for dialysis was placed. She was referred for SVC angioplasty to facilitate permanent dialysis access placement from the right IJV. Fig. 4.144 Complete “cup-shaped” SVC obstruction below azygos vein confluence with resultant azygos vein collateral circulation. The right IJV was accessed using ultrasound and a 6F vascular sheath was placed. The initial angiography showed complete SVC obstruction at a level just centrally to the azygos vein confluence, which resulted in remarkable dilatation and flow reversal of the azygos, making it the main collateral to the inferior vena cava (IVC) (Fig. 4.144). During attempted catheterization of the obstruction a straight-tipped stiff hydrophylic guidewire was advanced beyond the level of the obstruction (Fig. 4.145) but after catheter advancement and a brief hand injection it was discovered that the catheter was indeed in the pericardial cavity. The catheter was pulled back and a new angiography from the sheath proved there was apparent SVC dissection contrast media extravasation from the SVC into the pericardial cavity (Fig. 4.146). The patient stated she had difficulty breathing and chest pain, had a fall in blood pressure, and nonpalpable radial pulses. She also presented with distended neck veins. Low blood pressure and distended neck veins are recognized as the first two signs of the Beck’s triad for cardiac tamponade (the third being muffled heart sounds). Fig. 4.146 Digital subtraction angiography run from the sheath confirms SVC wall dissection beyond the level of the obstruction (black arrow) and site of wall rupture with extravasation into the pericardial cavity (white arrow). An upper abdominal ultrasound verified the presence of a large pericardial collection of blood about 4 cm in anterior thickness, presumably hemopericardium. Using a subxiphoid transhepatic approach, a 15-cm 18G needle was used to enter the pericardium. In the mean time an emergency anesthesiology team started resuscitating the patient. Blood aspiration from the pericardium using the 18G needle and 20-mL syringe suction proved to be inefficiently slow, so a standard J-type Tefloncoated guidewire was used (Fig. 4.147) to insert a 5F pigtail catheter. After removing about 200 mL of blood, again, due to insufficient drainage, the 5F pigtail was exchanged over the wire for a standard 8F pericardiocentesis set using a set of dilators. A total of approximately 500 mL of blood was drained from the pericardial cavity, with remarkable improvement in the patient. A completion angiography with the drainage tube in place showed no extravasation (Fig. 4.148). The drainage tube remained for 24 hours but with no additional drainage and was removed. A peritoneal dialysis catheter was then inserted, the femoral catheter removed, and the patient was discharged from the hospital. Fig. 4.147 Sonographically guided needle puncture and guidewire insertion into the pericardial cavity. Active bleeding from SVC catheter dissection and perforation to the pericardial cavity, creating acute pericardial tamponade. • Supporting the guidewire used to cross the obstruction with an inflated 16-mm balloon to center the crossing effort in the middle of the obstruction, could have prevented SVC dissection and perforation. The balloon could be also used as a safeguard, to inflate it in case SVC perforation occurs. It probably would have helped in this case to have an assistant inflate a balloon in the SVC while the primary operator tried to access the pericardium, but the presence of the resuscitation anesthesiology team allowed little room for sterile work around the patient’s IJV. Percutaneous sonographically guided placement of an 8F drainage tube in the pericardial cavity. Dissection across an SVC obstruction by the guide-wire and catheter along with perforation of SVC into the pericardial cavity, resulting in acute hemopericardium causing acute cardiac tamponade. • Be prepared and always have an appropriately sized balloon to inflate across a perforation. • Patients on dialysis are usually in a volume-depleted state, making them more prone to developing severe cardiac tamponade as a result of hemopericardium. Patients’ intravascular volume expansion and emergency team activation should be established immediately. • Plain needle aspiration in acute cardiac tamponade is insecure because it can cause loss of position, myocardial or coronary vessel injury, and is slow and cumbersome. It should be exchanged for a drain catheter as soon as possible. O’Horo SK, Soares GM, Dubel GJ. Acute pericardial effusion during endovascular intervention for superior vena cava syndrome: case series and review. Semin Interv Radiol 2007;24(1):82–86 Oshima K, Takahashi T, Ishikawa S, Nagashima T, Hirai K, Morishita Y. Superior vena cava rupture caused during balloon dilatation for treatment of SVC syndrome due to repetitive catheter ablation—a case report. Angiology 2006;57(2):247–249 Tempelhof M, Campbell J, Ilkhanoff L. Sinus arrest following angioplasty and stenting for superior vena cava syndrome. J Invasive Cardiol 2014;26(2):E21–E23 A 68-year-old patient suffered from critical ischemia of both legs. The patient had a previous aortobifemoral graft placed surgically 3 years ago, followed by surgical repair of a distal anastomotic pseudoaneurysm 4 weeks ago. The patient presented with acute critical leg ischemia of the right leg. Duplex ultrasound demonstrated an occluded right limb of the aortobifemoral graft. The patient underwent an emergent right axillary artery to right common femoral artery (CFA) surgical graft placement. The patient returned 3 weeks later with critical right leg ischemia and an occluded right axillary artery to CFA graft. The patient underwent a percutaneous endovascular attempt at recanalization of the axillary artery to CFA graft, which was unsuccessful. The procedure notes indicate an inability to cannulate the axillary artery to femoral artery graft from a right subclavian artery approach (Fig. 4.149). The patient subsequently developed swelling in the supraclavicular area on the right and presented to the Emergency Department 48 hours later. Computed tomography angiography (CTA). A large pseudoaneurysm arising from the right subclavian artery (Figs. 4.150 and 4.151). Fig. 4.149 Fluoroscopy showing contrast extravasation in the surrounding tissue; a guidewire and a catheter are still in place. Fig. 4.150 Contrast-enhanced computed tomography (CT) depicts a large pseudoaneurysm arising from the right subclavian artery. • Endovascular treatment via ipsilateral or contralateral retrograde access to the CFA with catheterization of the feeder vessel and placement of covered stent. • Embolization of pseudoaneurysm. • Thrombin injection into pseudoaneurysm. • Surgery (if an endovascular approach fails). Endovascular treatment via a right retrograde access to the CFA followed by insertion of a 90-cm-long 7F vascular sheath. Selective catheterization of right brachiocephalic artery using a 5F, 90°-angled catheter. Diagnostic angiogram demonstrated a large pseudoaneurysm arising from the mid-right subclavian artery (Fig. 4.152). The subclavian artery was crossed with an angled catheter and 0.035-inch, 260-cm-long Tefloncoated steel wire. A 7 × 40-mm covered stent was placed with complete exclusion of the pseudoaneurysm. There was no evidence of an endoleak (Fig. 4.153). Groin closure was performed using a “preclose technique” with use of two suture-mediated closure devices. Fig. 4.151 Coronal maximum intensity projection (MIP) reconstruction shows the large pseudoaneurysm arising from the mid-part of the right subclavian artery, located at suspected anastomosis site. Suspected uncontrolled advancement of a hydrophilic guidewire during the initial procedure resulted in perforation of right subclavian artery. • Guidewire advancement should be controlled under fluoroscopy, especially when using hydrophilic-coated wires! • Wires should never be pushed against resistance without fluoroscopic guidance. • Nonhydrophilic wires, if possible, when trying to cannulate or cross a vessel. Thompson CS, Rodriguez JA, Ramaiah VG, Olsen D, Diethrich EB. Pseudoaneurysm of the aortic arch after aortosubclavian bypass treated with endoluminal stent grafting—a case report. Vasc Endovasc Surg 2003;37(5):375–379 An 87-year-old woman with peripheral vascular disease presented with rest pain in her right leg. She was scheduled for recanalization of a distal superficial femoral artery (SFA)/popliteal artery occlusion (Fig. 4.154). Following antegrade access to the right common femoral artery (CFA) and insertion of a 6F sheath, the lesion was recanalized using a diagnostic catheter and a straight 0.018-inch hydrophylic guidewire V18 (Boston Scientific). The lesion was passed intraluminally and balloon angioplasty was performed (Fig. 4.155). During recanalization of the lesion the guidewire slipped into a collateral and the patient suddenly complained of sharp pain in her distal thigh/knee region. Angiography. There was perforation of a genual side branch from the guidewire, which had been advanced too far into the vessel in an uncontrolled fashion (Fig. 4.156).
4.1 Systemic Complication
Fatal retroperitoneal hemorrhage after antegrade locoregional thrombolysis
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Messag e
Further Reading
4.2 Access-Related Complications
4.2.1 Access Creation
Sudden groin swelling after successful 4F retrograde puncture
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Superficial femoral artery pseudoaneurysm and arteriovenous fistula
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Bleeding after failed antegrade groin access and PTA-related embolic event
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Hydrophilic-coated wire scaling during puncture needle manipulation (case 1)
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Hydrophilic-coated wire scaling during puncture needle manipulation (case 2)
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Complex iatrogenic femoral arterial access injury
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Bleeding complication due to an unrecognized side-branch perforation during retrograde groin access for PCI
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies Take-home Message
Further Reading
Rupture of a deep femoral artery side branch during diagnostic angiography
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
High common femoral artery puncture with sheath kinking
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Injury to the inferior epigastric artery during puncture
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Retrograde groin access complicated by external iliac artery dissection (case 1)
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Retrograde groin access complicated by external iliac artery dissection (case 2)
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Superficial femoral artery wire perforation during access creation
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
4.2.2 Access Closure
Stent elongation during vascular closure
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Insufficient common femoral artery access close after antegrade Angio-Seal application
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Impairment of walking capacity after retrograde Angio-Seal access closure
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Lack of clinical improvement after successful stent placement in the ipsilateral external iliac artery and retrograde Angio-Seal access closure
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Embolization of ExoSeal plug into the common femoral artery during antegrade groin closure
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
High-grade superficial femoral artery stenosis following cardiac catheterization
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Diffuse pelvic bleeding from expanding retroperitoneal hematoma
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Intravascular application of an Angio-Seal collagen plug
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Embolization of ExoSeal plug into the distal superficial femoral artery during antegrade groin closure
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
4.2.3 Intravenous Access
Port line dislocation into the right heart
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Port line dislocation into the right pulmonary artery
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Hemoptysis after subclavian vein catheterization in a patient with low platelets
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Puncture of the common femoral vein with external pudendal artery injury
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Port line migration during surgical explant
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Incidental insertion of a venous central line into the subclavian artery
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Central venous port line separation
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Venous access complication—fibrin sheath and adherent thrombus—and how to avoid embolic complications
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-Home Message
Further Reading
4.3 Lesion Treatment–Related Complications
4.3.1 Catheter Guidance and Navigation
Wire Advancement
Uncontrolled advancement of a hydrophylic 0.035-inch guidewire
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Hydrophylic guidewires and side-branch perforation (case 1)
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Clinical follow-up with inspection of the calf and Doppler assessment to rule out development of compartment syndrome.
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Hydrophylic guidewires and side-branch perforation (case 2)
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Flow-limiting axillary artery dissection after failed transbrachial approach for percutaneous coronary intervention
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Potentially fatal complication from superior vena cava angioplasty to facilitate permanent dialysis access
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Swelling of the supraclavicular area after unsuccessful recanalization of an occluded axillofemoral bypass graft
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Possible Strategies for Complication Management
Final Complication Management
Complication Analysis
Prevention Strategies and Take-home Message
Further Reading
Uncontrolled advancement of a hydrophilic 0.018-inch guidewire
Patient History
Initial Treatment Received
Problems Encountered during Treatment
Imaging Plan
Resulting Complication
Radiology Key
Fastest Radiology Insight Engine