(1)
Department of Nuclear Medicine, Kuwait University, Safat, Kuwait
12.2 Pathophysiology
12.2.1 Cerebrovascular Disease
12.2.2 Dementia
12.2.3 Epilepsy
12.2.4 Brain Tumors
12.2.5 Hydrocephalus
12.2.6 Cerebrospinal Fluid Leakage
12.2.7 Brain Death
12.3.1 Radiopharmaceuticals
12.3.3 Clinical Applications
Abstract
The central nervous system consists of the brain and the spinal cord. The major anatomical divisions of the brain are the cerebrum and the cerebellum, together weighing about 1,400 g in the adult. The cells in the brain are classified as glia or neurons. About 10,000 different types of neurons totaling approximately 100 billion neurons comprise the human brain. The cerebral cortex consists of two hemispheres connected by a large mass of white matter called the corpus callosum. The surface layer of each hemisphere is folded into gyri comprising the gray matter. The brain is divided into functional areas called the frontal lobe (anterior to the central sulcus) and the parietal lobe (posterior to this sulcus). The occipital lobe lies below the parieto-occipital sulcus, and the temporal lobe is situated below the lateral sulcus (Figs. 12.1 and 12.2). Knowledge of cross-sectional anatomy of the brain is a prerequisite for proper interpretation of brain imaging since tomographic imaging is the rule in current functional neuroimaging.
12.1 Anatomical and Physiological Considerations
The central nervous system consists of the brain and the spinal cord. The major anatomical divisions of the brain are the cerebrum and the cerebellum, together weighing about 1,400 g in the adult. The cells in the brain are classified as glia or neurons. About 10,000 different types of neurons totaling approximately 100 billion neurons comprise the human brain. The cerebral cortex consists of two hemispheres connected by a large mass of white matter called the corpus callosum. The surface layer of each hemisphere is folded into gyri comprising the gray matter. The brain is divided into functional areas called the frontal lobe (anterior to the central sulcus) and the parietal lobe (posterior to this sulcus). The occipital lobe lies below the parieto-occipital sulcus, and the temporal lobe is situated below the lateral sulcus (Figs. 12.1 and 12.2). Knowledge of cross-sectional anatomy of the brain is a prerequisite for proper interpretation of brain imaging since tomographic imaging is the rule in current functional neuroimaging.
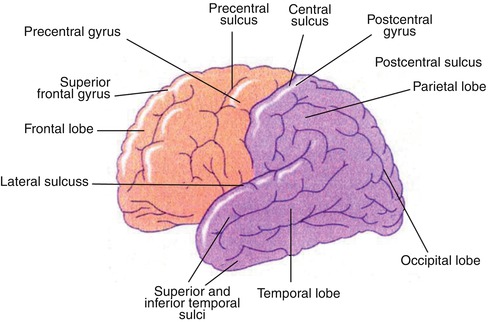
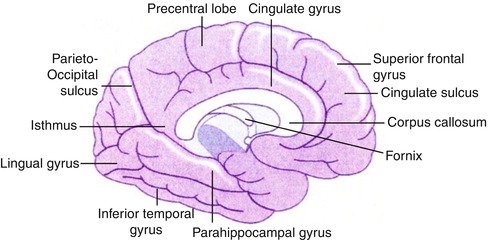

Fig. 12.1
Diagram of the lateral surface of the brain illustrating its main anatomical features

Fig. 12.2
Diagram of the brain illustrating the main internal structures
Neuronal blood flow utilization is related primarily to synaptic activity at the neuron cell body; thus, gray matter requires about four times as much blood flow as white matter. In the normal brain, the overall determinant of regional cerebral blood flow (rCBF) is dependent on vascular integrity, cerebral anatomy, and cerebral function. Since diseases of the brain can disrupt one or more of these functions, for accurate diagnosis it is important to integrate these three physiological functions with the pattern of rCBF change from normalcy to arrive at an accurate diagnosis of disease. In this chapter a review of radiopharmaceuticals commonly used to diagnose brain diseases is presented. This is followed by classes of disease which result in relatively specific patterns of abnormal tracer distribution, thus allowing for a specific diagnosis from the nuclear medicine scan.
Interpretation of scintigraphic brain studies depends on a background of neuroanatomy which with current techniques allow co-registration of MRI and CT with the functional images of SPECT and PET.
The intracranial and spinal cord structures float in CSF and are protected from jolts and blows. Principally, the choroid plexus in the lateral, third, and fourth ventricles produces the major portion of CSF. Normally, between 125 and 150 ml of CSF is circulating within the ventricles and subarachnoid space at any given time. Approximately 600 ml of CSF is produced daily. CSF is a clear fluid similar to blood plasma. The CSF normally drains from the lateral ventricles sequentially through the interventricular foramen of Monro, the third ventricle, and the cerebral aqueduct of Sylvius into the fourth ventricle and then leaves the ventricular system through the median foramen of Magendie and two lateral foramina of Luschka. Here the CSF enters the subarachnoid space. Along the base of the brain, this space extends into a number of lakes called cisterns. The CSF is absorbed through the pacchionian granulations of the pia-arachnoid villi into the superior sagittal sinus.
12.2 Pathophysiology
12.2.1 Cerebrovascular Disease
Cerebrovascular disease, also referred to as stroke, includes all disorders in which an area of the brain is temporarily or permanently affected by lack of blood flow or bleeding. Cerebrovascular diseases include stroke; carotid, vertebral, and intracranial stenoses; aneurysms; and vascular malformations.
The cause may be a blockage of the blood vessel, referred to as “ischemic stroke,” or due to a blood vessel which has burst, referred to as “hemorrhagic stroke.” Ischemic disease may cause stroke due to plaque rupture or pieces of plaque which have blocked a narrowed artery or when the person’s carotid artery has become completely blocked by plaque buildup. In hemorrhagic form, when a person’s blood vessel that supplies blood to their brain bursts, it prevents normal blood flow to their brain and permits blood to leak into an area of their brain, destroying tissue.
12.2.2 Dementia
Approximately three to four percent of the adult population in the United States demonstrates significant cognitive impairment. In general, the causes of dementia include primary neurodegenerative disorders with the most prevalent being Alzheimer’s disease, followed by frontotemporal dementia, Lewy body dementias, Parkinsonian dementia, progressive supranuclear palsy, Pick’s disease, cortical basilar degeneration, Huntington’s disease, and Wilson’s disease [1]. Vascular dementias are categorized as multi-infarct, Binswanger’s, and cerebral autosomal dominant arteriopathy with subcortical infarctions and leukoencephalopathy. Inflammatory etiologies include multiple sclerosis and vasculitis. Infectious etiologies include syphilis, human immunodeficiency virus, Lyme disease, and other viral disease and fungal diseases. Cancers are a rare cause of dementia which can be attributed to metastatic disease to the brain. Other causes and physical abnormalities include trauma and hydrocephalus.
It has been documented that approximately 80 % of all dementias is attributable to Alzheimer’s disease or Lewy body dementia. Vascular dementia comprises approximately 18 % of the dementias, with the other dementias less common [2].
12.2.2.1 Alzheimer’s Disease (AD)
AD is the most common cause of dementia in the elderly. AD is rarely familial. The sporadic form occurs usually after 65 years of age and accounts for most cases; it most likely results from a combination of genetic and environmental influences.
The only confirmed risk factors for sporadic AD are age and the presence of the E4 allele of APOE (apolipoprotein E). The brain of a patient with AD often shows marked atrophy, with widened sulci and shrinkage of the gyri. In the great majority of cases, every part of the cerebral cortex is involved; however, the occipital pole is often relatively spared. The cortical ribbon may be thinned and ventricular dilatation apparent, especially in the temporal horn, due to atrophy of the amygdala and hippocampus.
Microscopically, there is significant loss of neurons, in addition to shrinkage of large cortical neurons. The neuropathological hallmarks of AD include extracellular amyloid plaques and intracellular neurofibrillary tangles. The plaques are spherical structures consisting of a central core of fibrous protein known as amyloid that is surrounded by degenerating or dystrophic nerve endings. Neurofibrillary tangles are found inside neurons and are composed of paired helical filaments of hyperphosphorylated microtubule-associated tau protein. The intracellular deposition may cause disruption of normal cytoskeletal architecture with subsequent neuronal cell death. Neuritic plaques and neurofibrillary tangles are not distributed evenly across the brain in AD but are concentrated in vulnerable neural systems.
12.2.2.2 Other Causes of Dementia
Pick’s disease is a rapidly progressing frontal lobe type dementia. Primary progressive aphasia (PPA) is an uncommon type of degenerative dementia characterized by gradual impairment of language function that remains neuropsychologically focal for several years with sparing of the memory domain [3]. Compared with other neurodegenerative disorders that initially affect cognition followed by language impairment, many patients with PPA retain their cognitive functions allowing them to continue with their activities of daily living [3]. “Word-finding” or “naming” difficulty (dystonia) is the most common and earliest clinical presentation of PPA [4].
12.2.3 Epilepsy
Regional cerebral perfusion evaluation in patients with epilepsy has proven to be of significant clinical value for identification of the epileptogenic focus location. The underlying pathophysiology concerning the advantages of using regional cerebral perfusion tracers in epilepsy is based on the clinical observation of an increase in cortical blood flow in the area of seizure discharge on surgery. Therefore, the most valuable use of 99mTc-HMPAO evaluation of the epilepsy patient is to localize the epileptogenic focus during the ictal state.
12.2.4 Brain Tumors
Brain tumors may be primary or metastatic. Primary tumors are classified by the type of tissue in which they arise. Gliomas come from glial cells such as astrocytes, oligodendrocytes, and ependymal cells. The gliomas, accordingly, include three types:
Astrocytic tumors (astrocytomas) may grow anywhere in the brain or spinal cord. In adults, astrocytomas most often arise in the cerebrum. In children, they occur in the brain stem, the cerebrum, and the cerebellum. A grade III astrocytoma is sometimes called anaplastic astrocytoma. A grade IV astrocytoma is usually called glioblastoma multiforme (the most aggressive type of all primary brain tumors).
Oligodendroglial tumors (oligodendrogliomas) arise in the cells that produce myelin which is the fatty covering that protects nerves. The tumors usually arise in the cerebrum. They grow slowly and usually do not spread into surrounding brain tissue. Ependymomas usually develop in the lining of the ventricles. They may also occur in the spinal cord. Some primary brain tumors are made up of both astrocytic and oligodendrocytic tumors. These are called mixed gliomas.
Meningiomas arise from the meninges. They are usually benign. Because these tumors grow very slowly, the brain may be able to adjust to their presence; meningiomas may grow quite large before they cause symptoms. Schwannomas are benign tumors that grow from Schwann cells, which produce the myelin that protects peripheral nerves. Acoustic neuromas are a type of schwannoma. They occur mainly in adults. These tumors affect women twice as often as men. Other rare tumors are craniopharyngiomas, pituitary tumors, primary CNS lymphoma, pineal gland tumors, and primary germ cell tumors of the brain. Most primary brain tumors do not metastasize, but if they do metastasize, intracranial spread generally precedes distant spread.
Metastatic brain tumors are five times more common than primary brain tumors. The most common types of cancer that cause metastatic brain tumors are lung cancer, breast cancer, melanoma, colon cancer, renal cell carcinoma, and thyroid cancer. Metastatic brain tumors from non-CNS primary tumors may be the first sign of malignancy, or they may herald a relapse. Nonetheless, the signs and symptoms of brain metastases simulate those of primary brain tumors.
Brain tumors, primary or metastatic, produce neurological manifestations by several mechanisms. Tumors can invade and infiltrate normal parenchymal tissue, disrupting normal function. Since brain is contained in limited volume of the cranial vault, growth of intracranial tumors with accompanying edema may cause increased intracranial pressure. Tumors may generate new blood vessels (angiogenesis), disrupting the normal blood-brain barrier and promoting edema. Small, critically located tumors may damage specific neural pathways traversing the brain. Tumors located adjacent to the third and fourth ventricles may impede the flow of cerebrospinal fluid causing obstructive hydrocephalus.
The cumulative effects of tumor invasion, edema, and hydrocephalus may elevate the intracranial pressure which impairs cerebral perfusion. Compartmental rise of intracranial pressure may lead to shifting or herniation of tissue under the falx cerebri, through the tentorium cerebelli, or through the foramen magnum. Leptomeningeal infiltration may present with dysfunction of multiple cranial nerves.
12.2.5 Hydrocephalus
The term hydrocephalus generally refers to those conditions that produce an imbalance between the rate of production and absorption of the cerebrospinal fluid, leading to dilatation of the ventricular system. Hydrocephalus normally occurs as a result of obstruction to the flow and absorption of CSF, although there are rare cases of choroid plexus papillomas causing hydrocephalus by overproduction of CSF.
Hydrocephalus is traditionally classified as communicating and noncommunicating, based on whether ventricular obstruction is present. In the former, the ventricular system continues to communicate with the subarachnoid spaces outside the brain through the fourth ventricular foramina of Luschka and Magendie. Noncommunicating hydrocephalus correspondingly refers to the presence of occlusion within the ventricular system. Hydrocephalus may be either congenital or acquired. Arnold-Chiari malformation, Dandy-Walker malformations, and aqueductal stenosis/atresia are common causes of the congenital variety. In the acquired type, many pathological conditions, including inflammatory, infectious, traumatic, and neoplastic disorders, can cause hydrocephalus [5, 6].
Noncommunicating hydrocephalus can be the result of intraventricular mass, aqueductal obstruction, or fourth ventricular obstruction. Communicating hydrocephalus, on the other hand, results from meningitis, meningeal carcinomatosis, or cerebral dural sinus thrombosis, or it is idiopathic in elderly patients. Normal-pressure hydrocephalus (NPH) is a communicating hydrocephalus of particular interest to nuclear medicine professionals since radionuclide cisternography is useful in its diagnosis and management.
In NPH, the usual flow of CSF is impaired somewhere in the intracranial subarachnoid space, resulting in a reversal of CSF flow and dilatation of the lateral ventricles. There is free communication between the ventricular system and the subarachnoid pathways and no elevation of CSF pressure. Clinically, the entity occurs in patients 50–70 years of age and is characterized by dementia, gait disturbances, and fecal and urinary incontinence. Most commonly, this condition results from subarachnoid hemorrhage or meningoencephalitis.
12.2.6 Cerebrospinal Fluid Leakage
Leaking of CSF may be etiologically classified into:
1.
Traumatic: occurring in about 30 % of basilar skull fractures. Two percent of all head injuries develop a CSF fistula. This leak is usually unilateral, scanty, and seen within 48 h after trauma and resolves in 1 week.
2.
Nontraumatic: taking place in tumors (pituitary, brain, and skull), skull infections, and congenital defects (encephalocele). This leak is profuse and may persist for years. Infection complicates the untreated leak in 25–50 % of cases.
12.2.7 Brain Death
Death is an irreversible, biological event that consists of permanent cessation of the critical functions of the organism as a whole. It implies permanent absence of cerebral and brain stem functions. Brain death then qualifies as death (as brain is essential for integrating critical functions of the body). The equivalence of brain death with death is largely but not universally accepted.
The concept of irreversible coma or brain death was first described in 1959, predating widespread organ donation. The fundamental definition of brain death has substantively remained constant over time and across countries (although specific details of diagnostic criteria differ). Many conditions are known to cause brain death (Table 12.1).
Table 12.1
Causes of brain death
Trauma and subarachnoid hemorrhage: most common |
Intracerebral hemorrhage |
Hypoxic ischemic encephalopathy |
Ischemic stroke |
Any condition causing permanent widespread brain injury |
12.3 Scintigraphic Evaluation of CNS Diseases
12.3.1 Radiopharmaceuticals
In addition to 111In-DTPA and 99mTc-DTPA used for CSF imaging (radionuclide cisternography), there are three main classes of radiopharmaceuticals available for functional brain imaging in nuclear medicine.
1.
Regional cerebral blood flow agents
SPECT radiopharmaceuticals used for measuring regional cerebral blood flow (rCBF) are lipophilic agents which are transported from the arterial vascular compartment to the normal brain tissue compartment by diffusion and are distributed proportional to regional tissue blood flow. After this first phase of transport, the tracers are essentially irreversibly trapped in the tissue compartment. The two major blood flow agents used in brain SPECT imaging are technetium-99 m hexamethylpropylene amine oxime (99mTc-HMPAO) and technetium-99 m ethyl cysteinate dimer (99mTc-ECD) [9–11]. Xenon-133(133Xe) is unique since it is freely diffusable and not trapped in the tissues. Inhaled or i.v. injection of 133Xe dissolved in saline can more accurately and quantitatively provide measurements of blood flow by determination of the clearance rate of this tracer from the cerebral compartment, after a brief uptake period [12]. The major PET radiopharmaceutical used to measure cerebral perfusion is 15O H2O [5].
2.
Regional cerebral metabolism agents
These radiopharmaceuticals are transported to the brain tissues by regional cerebral blood flow, but subsequent regional cerebral distribution reflects the utilization rate of the tracer in a cerebral metabolic pathway. Currently, there are no SPECT tracers that specifically measure normal cerebral metabolism. However, in brain tumor imaging, where the blood–brain barrier is broken, ionic tracers such as thallium-201 [13] or other SPECT tracers such as 99mTc- methoxyisobutylisonitrile (99mTc-MIBI) [14] can be used to detect new, recurrent, or residual viable tumor. The PET radiopharmaceutical predominantly used is fluorine-18 2-fluoro-2-deoxy-d-glucose (18F-FDG) [15]. [18F]-fluoro-3′-deoxy-3′-l-fluorothymidine (FLT) is a newer tracer used to indicate tumor proliferation to more specifically identify new, recurrent, or residual viable brain tumor.
3.




Central nervous system receptor-binding agents
This class of radiotracers important in brain imaging is central nervous system receptor-binding agents, which measure neuronal receptor density and binding affinity [16]. In SPECT, a dopamine transporter receptor-binding agent which has been well characterized is 123I-ioflupane. This benzamide compound has been used to image the dopaminergic (D2) transporter system in the corpus striatum [10].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree