CHAPTER 36 Cerebellopontine Angle and Internal Auditory Canal Neoplasms
The cerebellopontine angle (CPA) cistern is a cerebrospinal fluid (CSF)–filled space whose boundaries are made up by the pons and cerebellum medially, the petrous portion of the temporal bone laterally, and the tentorium superiorly. The Cranial nerves V through VIII pass through the upper portion of the cistern, whereas the cranial nerves IX through XI pass through the lower portion. The presenting symptoms of CPA lesions are usually not related to the histology of the lesion but to the nerves and other structures that the lesion affects.
Neoplasms occurring in this region make up 6% to 10% of all intracranial tumors in adults and only 1% of lesions in children.1,2 The two most common lesions are the meningioma and the vestibular schwannoma. Indeed, meningiomas and schwannomas comprise approximately 90% of neoplasms in this region.3 However, a variety of other lesions also make their home in the CPA region. These lesions arise within the cerebellopontine cistern or from related structures and include arachnoid cysts, nonacoustic schwannomas, and meningeal lesions. Embryologic remnants may also be found, including lipomas, epidermoid cysts, and neurenteric cysts. Lesions, such as chondromatous tumors, chordomas, paragangliomas, and endolymphatic sac tumors, involve the CPA by extension from surrounding structures such as the skull base and petrous apex. Exophytic brain stem neoplasms or ventricular tumors can also involve the CPA. Locating a point of origin along with knowledge of a lesion’s morphology, CT density, MR intensity, and reaction of adjacent structures can help narrow the differential diagnosis.
SCHWANNOMA
Epidemiology
Vestibular schwannomas are the most common lesions of the CPA, comprising up to 90% of lesions that occur within this region. For sporadic vestibular schwannomas, the age at presentation is typically between 30 and 40 years of age. In patients with neurofibromatosis type 2 the presentation is frequently earlier, usually in the second decade of life. Some reports describe no sex predilection; however, others report an increased prevalence in women of 2 : 1.1,4
Pathophysiology
In the majority of cases (>95%), schwannomas arise as sporadic lesions. However, schwannomas are also associated with neurofibromatosis type 2, which is an autosomal dominant syndrome with a mutation located on chromosome 22. These patients frequently have multiple schwannomas, and the presence of bilateral vestibular schwannomas is nearly pathognomonic for the disorder. In addition, these patients also have a propensity to develop meningiomas and ependymomas (Fig. 36-1).
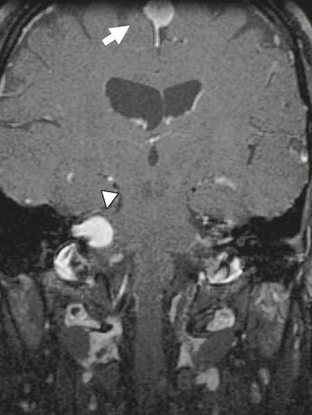
FIGURE 36-1 Neurofibromatosis type 2. Coronal T1W MR image after gadolinium administration demonstrates an enhancing lesion within the right IAC (arrowhead) consistent with a vestibular schwannoma. In addition, a meningioma (arrow) is noted adjacent to the falx cerebri.
Pathology
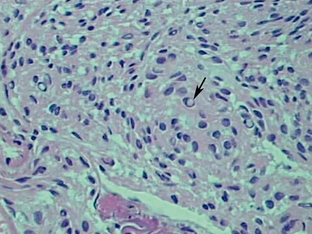
Schwannomas are well-circumscribed lesions that are most often tan (Fig. 36-2). The larger and older tumors may undergo benign cystic degeneration. They are spindle cell neoplasms that are composed of two tissue types, the Antoni A and Antoni B cells (Fig. 36-3). The Antoni A regions are hypercellular and compactly arranged. These areas account for a lower signal on T2-weighted (T2W) images. Antoni B regions are composed of looser myxomatous tissue, resulting in a brighter signal on T2W images. These areas are thought responsible for cystic changes within schwannomas. The imaging findings of schwannomas are dependent on the component of Antoni A or B cells. Antoni A and B tissues may align into palisades, which are referred to as the Verocay body.5
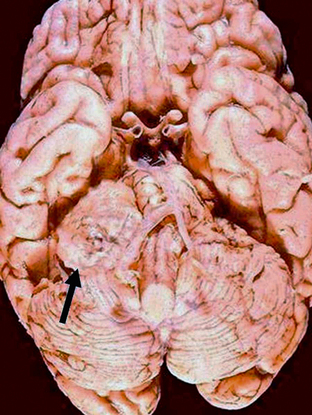
FIGURE 36-2 Vestibular schwannoma. Gross specimen of the brain demonstrates a mass (arrow) with similar coloration to the adjacent brain that is displacing the brain stem.
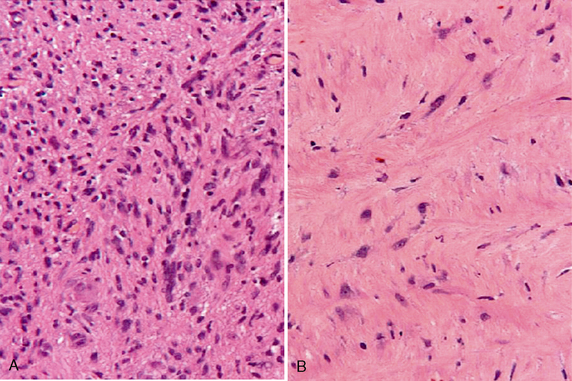
FIGURE 36-3 Schwannoma. Antoni A cells (A) stain more darkly and are more compact than the more loosely arranged Antoni B cells (B).
Immunohistochemical staining of schwannomas is positive for S-100, which is a protein found in cells derived from neural crest origin. They may be focally positive for glial fibrillary acidic protein (GFAP). GFAP is a member of the intermediate filament family that provides support and strength to astroglial cells.5
Imaging
CT
For the most part, MRI has replaced CT in the evaluation of CPA angle masses. When CT is utilized, thin-section (1.5 mm) imaging should be performed. The imaging characteristics of schwannomas on CT depend in part on the size of the lesion. Smaller schwannomas may not be readily visible on CT. In the case of vestibular schwannomas a small lesion arising along the intracanalicular portion of the nerve will be contained entirely within the internal auditory canal (IAC) and may not be readily visible by CT. As the lesion enlarges, it extends outward into the CPA and presents as a rounded mass that is either hypoattenuating or isodense to brain. This is referred to by some as the “ice cream cone” pattern. Expansion of the IAC may be seen in up to 70% to 90%.6 In addition, the intracanalicular lesions may “dumbbell” into the vestibule or cochlea, which is better appreciated on MRI.7
Larger lesions may have a heterogeneous appearance due to cystic degeneration. In fact, most, if not all, schwannomas larger than 2.5 cm become heterogeneous due to cystic and necrotic components.8 On postcontrast imaging schwannomas demonstrate enhancement. Calcification is very rare within schwannomas and, if seen, should raise the suspicion of a meningioma. Thin-section (1.5 mm) imaging utilizing bone algorithm assists in visualizing the expansion of the IAC as well as any small calcifications within the lesion.
MRI
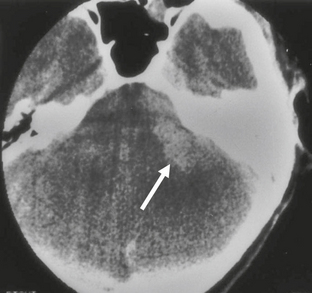
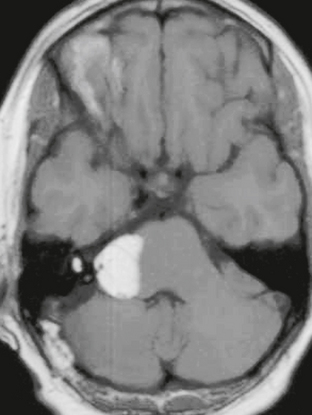
The appearance of schwannomas on MRI, like CT, may vary with the tumor size. As the lesions become larger they may undergo cystic change, and this alters their signal characteristics. Typically, schwannomas are slightly hypointense to isointense on T1-weighted (T1W) imaging. However, T1 hyperintensity may be seen if there has been hemorrhage into the schwannoma or if regions of cystic degeneration contain proteinaceous material. These lesions enhance intensely after gadolinium infusion, and the degree of homogeneous enhancement depends on the amount of cystic change (Fig. 36-4). Schwannomas are typically hyperintense on T2W imaging, and if cystic degeneration is present more focal areas of increased signal will be seen. Expansion of the IAC may be seen in up to 70% to 90% of vestibular schwannomas (Fig. 36-5).6 Approximately 5% of schwannomas may be associated with an arachnoid cyst that occurs between the neoplasm and the brain, suggesting that the mechanism of formation is peritumoral adhesions (Fig. 36-6).9
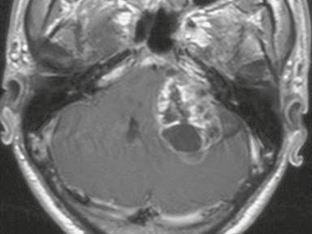
FIGURE 36-4 Vestibular schwannoma. A large, heterogeneously enhancing mass is seen in the right CPA. Areas of nonenhancement correspond to regions of cystic degeneration.
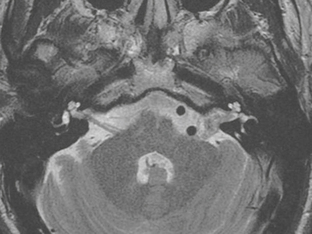
FIGURE 36-5 Vestibular schwannoma. Axial T2W MR image demonstrates a heterogeneous-appearing lesion involving the left IAC that extends into the CPA. Widening of the left IAC is noted.
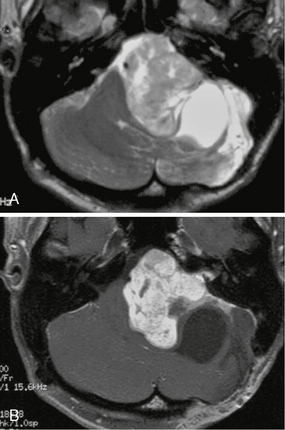
FIGURE 36-6 Schwannoma and arachnoid cyst. Axial T2W (A) and T1W postcontrast (B) MR images through the CPA reveal a large vestibular schwannoma with a focal, well-circumscribed nonenhancing area of T2 hyperintensity posterior and lateral to the lesion consistent with an arachnoid cyst.
The major imaging differential diagnosis for schwannomas is the meningioma. Meningiomas may extend into the IAC and may be very difficult to distinguish from a schwannoma. However, the ability to center the lesion over the IAC greatly favors schwannoma. In general, schwannomas are usually more hyperintense on T2W images compared with meningiomas. They also tend to be more spherical as opposed to meningiomas, which are more often hemispheric. Meningiomas may occasionally result in expansion of the IAC, but this is much less common than with schwannomas.
If the lesion is large enough, MR spectroscopy has the potential to aid in differentiating the two lesions. Schwannomas have been noted to have elevated signal at 3.56 ppm representing myoinositol, as well as reduction in N-acetyl-aspartate (NAA) and elevation in choline. Meningiomas, on the other hand, demonstrate absence of NAA because they are not of neural origin, and an alanine peak may be noted at 1.55 ppm.10 Evaluation of the brain stem with a 3D fast spin-echo heavily T2W sequence may reveal a focus of increased signal intensity in the dorsal brain stem in the region of the vestibular nucleus unilateral to the lesion. This is thought to represent degeneration of the vestibular nucleus associated with a vestibular schwannoma and helps to suggest the diagnosis.11 Relative cerebral blood volume (rCBV) has been evaluated as a means of differentiating meningiomas from schwannomas; however, there is some overlap between the two lesions. Studies have compared the rCBV ratios (rCBV of the lesion divided by the rCBV of normal white matter) and found the highest reported rCBV ratio in schwannomas to be 4.4, whereas in meningiomas the ratio typically ranges from 6 to 9.12,13
MENINGIOMA
Epidemiology
Meningiomas comprise 10% to 15% of CPA masses. These lesions are more frequent in women, and the gender ratio ranges from 2 : 1 to 4 : 1 depending on the series. Meningiomas typically occur between the ages of 20 and 60 years, but these lesions have been found in children.1,14,15 They are also found in association with neurofibromatosis type 2, in which case they are frequently multiple.
Pathophysiology
Meningiomas arise from meningothelial cells, which are also known as arachnoid cap cells. The World Health Organization (WHO) grades meningiomas from I to III. The most frequent form is grade I, which has a 7% to 20% recurrence rate. Grade II recurs in 29% to 40%. Grade III are highly aggressive, rapidly growing meningiomas that have a 50% to 78% recurrence rate. Twenty percent of meningiomas display aggressive histologic features, and the pathologic criteria for a higher grade include hypercellularity, loss of architecture, nuclear pleomorphism, mitotic index, tumor necrosis, and brain invasion. Metastasis can be seen in approximately 0.1% of meningiomas. Higher-grade meningiomas are more common in males and children.16
Immunohistochemistry is beneficial in making the diagnosis of meningiomas. In 80% of cases meningiomas are positive for epithelial membrane antigen. Interestingly, progesterone receptors can be found in the cytoplasm of meningiomas and may play a role in the proliferation of meningiomas in females. However, there is debate in the literature of whether these progesterone receptors are functional.17
Pathology
Meningiomas are well-demarcated lesions with a broad dural base (Fig. 36-7). They are firm, rubbery lesions whose appearance varies depending on the degree of cystic change, lipid content, calcification, and vascularity. Meningiomas also frequently stimulate hyperostosis in adjacent bone.
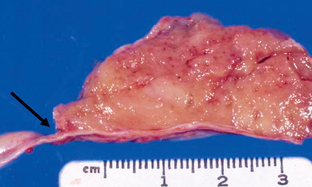
FIGURE 36-7 Meningioma. Gross specimen demonstrates a well-circumscribed, hemispheric lesion with a broad base and dural attachment (arrow).
Meningiomas are composed of monomorphic cells and have oval nuclei that may demonstrate intranuclear inclusions (Fig. 36-8). Psammoma bodies, which are rounded collections of calcium, may be seen interspersed between the cells. The three most common histologic subtypes of meningiomas are meningothelial, fibroblastic, and transitional. The meningothelial form consists of densely packed cells arranged in sheets. Fibroblastic meningiomas consist of densely packed sheets of spindle cells interwoven with collagen and reticulin. Transitional meningiomas have features common to both the fibroblastic and meningothelial forms.1
Imaging
CT
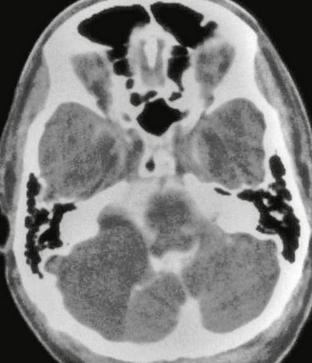
Meningiomas are usually homogeneously hyperattenuating, well-circumscribed, extra-axial lesions on CT. The center of the mass tends to be eccentric to the porus acusticus. Very rarely are they located entirely within the IAC. They are vascular lesions and, typically, homogeneously and intensely enhance after contrast agent administration. Fifteen to 20 percent will demonstrate calcification. Hyperostosis may be seen, which is not related to the presence of bone invasion, and is more likely related to local hypervascularity (Fig. 36-9).18 In fact, CT may be of benefit when evaluating for a small meningioma because hyperostosis may be the only indication of its presence. However, detection of hyperostosis in the petrous bone may be more difficult than in other locations owing to the inherent density of this bone.
MRI
Meningiomas are hypointense to isointense on T1W imaging and avidly and homogeneously enhance on postcontrast imaging owing to their vascular nature. They are isointense to hyperintense on T2W imaging (Fig. 36-10), but there may be areas of hypointensity that correlate to regions of calcification. T2 hyperintensity may be seen within the adjacent brain parenchyma secondary to tumor-induced vasogenic edema. On postcontrast imaging a “dural tail” may be seen, but this is not pathognomonic for meningiomas and has been reported in other lesions adjacent to the dura, including both schwannomas and hemangiopericytomas. In addition, the dural tail is only seen in up to 60% of meningiomas.19
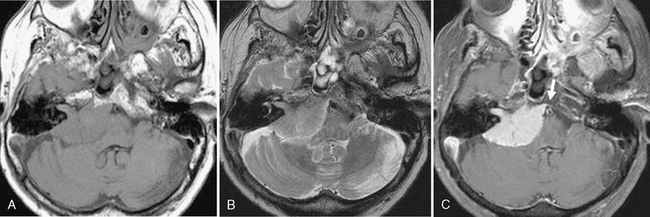
FIGURE 36-10 Meningioma. A, Axial T1W MR image demonstrates a well-circumscribed lesion of the CPA that is isointense to brain and results in mass effect on the adjacent medulla and cerebellum. B, Axial T2W MR image demonstrates that the signal of the lesion is again relatively isointense to that of brain. C, On postcontrast imaging the lesion homogeneously enhances and a small dural tail is noted (arrow). The contrast agent helps to outline the extent to which the lesion involves the IAC.
Atypical imaging features such as heterogeneous enhancement, cyst formation, hemorrhage, and fatty degeneration may be seen in approximately 15% of histologically benign meningiomas.18 The degree of associated vasogenic edema does not appear to correlate with tumor grade or size. The etiology of the vasogenic edema is controversial and is probably the result of a combination of different mechanisms.18
MR perfusion demonstrates an elevated rCBV ratio, usually in the range of 6 to 9. Typically, there is less than 50% return to baseline because of the extra-axial nature of these lesions. This marked elevation in rCBV may be of some assistance in differentiating meningiomas from schwannomas, whose rCBV ratio values typically do not exceed 4, but there can be overlap.12 Because of susceptibility artifact in the posterior fossa, obtaining perfusion studies in this region may be limited. MR spectroscopy may demonstrate an alanine doublet at 1.4 ppm, but, again, like perfusion, there may be limitations of spectroscopy in the posterior fossa owing to the surrounding osseous structures. Alanine is found in tumors of meningeal origin and may be seen in 30% to 40% of meningiomas. NAA is not visualized because these tumors are not of neural origin, and choline may be elevated due to rapid membrane turnover.20
Special Procedures
On angiography, meningiomas have a tumor blush that appears early and persists to the venous phase. This appearance is often jokingly referred to as the “mother-in-law” effect. One or more large meningeal vessels are typically noted feeding the meningioma. Preoperative angiography is beneficial to identify the vascular supply and embolization may be of benefit to reduce bleeding during surgery.
EPIDERMOID CYST
Epidemiology
Epidermoid cysts make up fewer than 5% of intracranial masses; however, they are the third most common mass lesion in the CPA. They typically present in the third to fourth decades.21
Clinical Presentation
These cysts grow slowly and may produce only mild symptoms as a result of local mass effect. Most frequently these symptoms consist of a chronic headache or facial pain. Although very rare, brain stem stroke resulting from stretching of the basilar artery has been reported.22
Pathophysiology
Epidermoid cysts are remnants of ectodermal tissue misplaced during embryogenesis that may result from failure of the surface ectoderm to separate from underlying structures, from implantation of surface ectoderm, or from sequestration of surface ectoderm. This probably occurs at 3 to 5 weeks of gestational age when the neural tube is closing.21
Pathology
Epidermoid cysts are unilocular lesions. They expand over decades as a result of accumulation of debris from the desquamation of the lining. They tend to be shiny, smooth, and waxy and are frequently described as “pearly” (Fig. 36-11). Scattered calcifications may be seen along the surface.
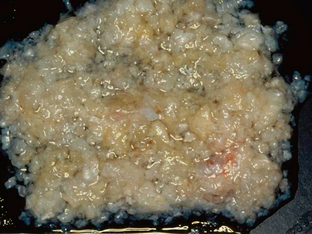
FIGURE 36-11 Gross image of an epidermoid cyst reveals the “pearly” appearance characteristically described in these lesions.
Epidermoid cysts are composed of an internal layer of squamous epithelium that is covered by an external fibrous capsule. They are filled with keratinous material that typically forms layers, giving an “onionskin” appearance (Fig. 36-12).
Imaging
CT
Epidermoid cysts are well-demarcated, unilocular lesions. The margins are typically scalloped and irregular due to molding around adjacent structures. On CT they are hypodense, nonenhancing mass lesions that have attenuation similar to CSF. The hypodensity is thought to be due to the high cholesterol and keratin content of the desquamated debris. Occasionally, they may have slightly lower attenuation numbers (−10 to −20), but these numbers are never low enough to be confused with fat. Even rarer, they may be hyperdense, which, like bronchogenic cysts, may be related to high protein content.23 Hemorrhage within the cyst and elevated protein content are both suggested causes for this. On postcontrast imaging, epidermoid cysts typically do not enhance. On occasion, thin peripheral enhancement may be seen along the lining that may be due to an inflammatory response. The lobulated, irregular margins help distinguish them from arachnoid cysts, which usually have smooth margins. Epidermoid cysts tend to insinuate themselves around structures rather than displacing them; however, they may become adherent to these structures, making resection difficult.
MRI
MRI of epidermoid cysts usually demonstrates T1 and T2 intensity that is similar to CSF. However, high T1 signal intensity has been reported in some epidermoid cysts (“white epidermoids”). These lesions have a high lipid content composed of mixed triglycerides that contain unsaturated fatty acid residues.24 Occasionally, low signal may be seen on T2W images that may be related to elevated protein content and increased viscosity, and these may also have increased signal on T1W images. Hemorrhage into arachnoid cysts has been reported, which alters the signal characteristics.25 On fluid-attenuated inversion recovery (FLAIR) imaging, epidermoid cysts do not follow CSF, and they demonstrate reduced diffusion on diffusion-weighted imaging (Fig. 36-13). The finding of reduced diffusion is useful in postsurgical follow-up to assess for the presence of residual material. Both the FLAIR signal and reduced diffusion help to differentiate epidermoid cysts from arachnoid cysts, which follow CSF signal on all sequences. Unlike arachnoid cysts, epidermoid cysts tend to engulf nerves and vessels, rather than displace them, which can make surgical resection difficult. On MR spectroscopy, elevated lactate levels are seen within epidermoid cysts.26
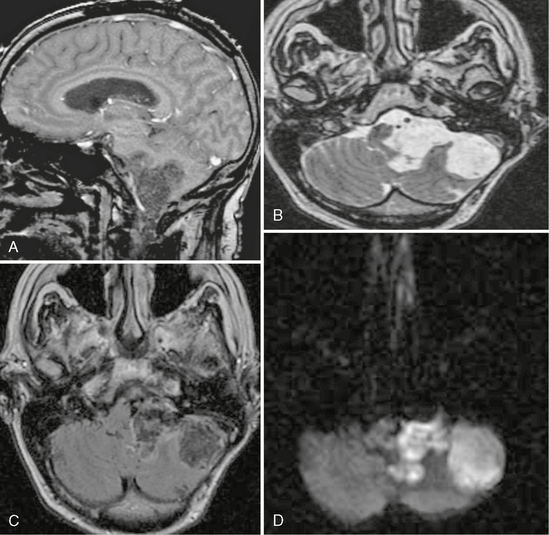
FIGURE 36-13 Epidermoid cyst. A, Sagittal T1W SPGR MR image demonstrates an irregularly marginated lesion in the region of the CPA. B, Axial T2W MR image again demonstrates the irregular margins. The signal is similar to CSF, but there is the subtle appearance of lamellation within the lesion. C, On the axial FLAIR image the signal does not completely saturate as would be expected with CSF. D, The diffusion-weighted image demonstrates high signal, which helps to confirm the diagnosis of an epidermoid.
LIPOMA
Lipomas are non-neoplastic developmental lesions developing from the meninx primitiva, which is the precursor to the pia mater and arachnoid.
Imaging
CT
On CT, lipomas are low-attenuation lesions with negative attenuation values (−50 to −100) consistent with fat (Fig. 36-14). They do not demonstrate any internal contrast enhancement. CPA lipomas may be difficult to detect owing to beam hardening in this region.
ARACHNOID CYST
Arachnoid cysts are intra-arachnoid “pouches” that are filled with CSF.
Epidemiology
Arachnoid cysts comprise approximately 1% of intracranial lesions, but the exact incidence is unknown because many are asymptomatic throughout life and may go undetected. When they are symptomatic, they typically present in the first 2 decades of life. There is no known gender predilection.28 Approximately 10% of arachnoid cysts are located in the posterior fossa. The remainder are located in the middle cranial fossa and suprasellar cistern.
Pathophysiology
Rarely, hemorrhage can occur into the arachnoid cyst. This may be secondary to trauma. It is possible that even mild trauma may result in disruption of unsupported vessels surrounding the arachnoid cyst resulting in an increase in size and symptoms of mass effect due to bleeding.29 Hemorrhage can result in enlargement of the cyst. Other causes of enlargement are thought to be due to the possibility of passive fluid diffusion into the cyst or a ball-valve mechanism. Active secretion from the cyst wall has also been speculated, but this is controversial.30
Pathology
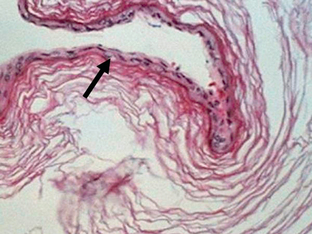
Arachnoid cysts are well-marginated cysts with smooth walls that contain CSF (Fig. 36-16).
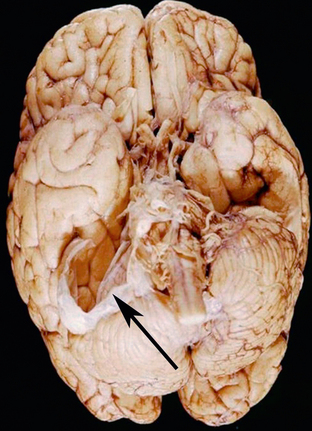
FIGURE 36-16 Arachnoid cyst. Gross specimen reveals a membrane (arrow) that had surrounded an arachnoid cyst within the CPA. Distortion of the cerebellar hemisphere is noted from the mass effect that was exerted by the cyst.
Imaging
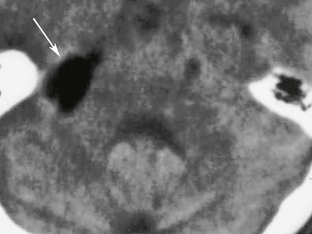
CT
CT cisternography can be utilized to help distinguish between communicating and noncommunicating cysts. This involves the intrathecal administration of a contrast agent and evaluation of the presence of contrast agent within the cyst (Fig. 36-17). If no agent is initially seen, then delayed imaging is performed.