Julius Chapiro • Rafael Duran • Jean-François Geschwind
Primary liver cancer is the third most common cause of cancer-related death worldwide. Asia continues to be the region with the most cases of hepatocellular carcinoma (HCC), but increasing incidences have been reported in Europe and North America, primarily due to the concomitant rise in hepatitis C infection.1 As most patients are diagnosed at advanced stages, therapeutic options remain limited and represent an oncologic challenge with a dismal prognosis. Over the past three decades, the importance of catheter-based intra-arterial therapies in the management of primary liver cancer patients with more advanced disease (intermediate to advanced) has continued to grow. This trend is mainly due to their tremendous advantages over systemic chemotherapy. The dual benefit of reduced systemic toxicity combined with effective local tumor control helped to include some procedures into the National Comprehensive Cancer Network treatment guidelines. Moreover, the success of intra-arterial therapies contributed to the formation of interventional oncology, a new subdiscipline within interventional radiology, unifying specialists in multidisciplinary teams of physicians from multiple specialities bound by the same desire to tackle primary liver cancer.2 In this regard, transarterial chemoembolization (TACE) is one of the most frequently used and most exhaustively studied modalities.
A fairly recent development of advanced drug delivery systems such as drug-eluting beads (DEBs) provided an additional option for controlling drug release to tumors and paved the way for the DEB-TACE procedure. This technique was introduced to the majority of interventional radiologists less than 10 years ago. In DEB-TACE, the oily medium used for conventional TACE (cTACE) has been substituted for a polymer-based microsphere, which results in enhanced drug delivery to the tumor and further reduction in systemic drug exposure.3 As a result, there has been a growing interest in using DEB-TACE to treat patients with HCC, and some trials combine this modality with systemically administered targeted agents to maximize the benefits of both therapies. This development has led to a shift away from cTACE toward DEB-TACE in the treatment of patients with HCC, especially in the United States and Europe.3,4 In this chapter, we will describe the scientific rationale for DEB-TACE, discuss materials and techniques used for this procedure, and show the most recent clinical evidence for safety and efficacy of this modality. Finally, we will touch on the combination of DEB-TACE with systemically administered targeted agents.
MATERIALS
The general concept of TACE was originally introduced by Yamada et al.,5 who exploited HCC’s preferential blood supply from the hepatic artery for the local delivery of chemotherapy. The advent of DEBs further improved on the ability of cTACE to deliver higher concentration of drugs directly to liver tumors while significantly reducing the serum concentration of chemotherapy and thereby minimizing systemic toxicities.6 Two major types of drug-eluting microsphere, LC Beads (DC Beads in Europe; Biocompatibles UK Ltd., Farnham, Surrey, United Kingdom) and QuadraSpheres (HepaSpheres in Europe; Merit Medical Systems, Inc., South Jordan, Utah), are available clinically in the United States. However, neither of the products is approved as a drug-delivery microsphere by the U.S. Food and Drug Administration (FDA) but rather as embolic material.
Most of the clinical data has been generated with the LC Beads. These beads are made of nonbiodegradable materials, such as polyvinyl alcohol (PVA) hydrogel that has been modified by the addition of sulfonic acid–containing components. The microspheres are soft, compressible, spherical particles that can be loaded with doxorubicin or irinotecan.7,8 When polymerized, they can formulate different sizes, ranging from 75 to 900 µm. The smaller bead diameters achieve a more distal embolization and more extensive necrosis as compared with larger beads and are more commonly used.9 The manufacturer defines the maximum loading capacity for doxorubicin as 40 mg/mL hydrated LC Beads, yet a loading of 25 mg/mL was recommended out of technical considerations.10 Pharmacokinetic studies have demonstrated that drug elution occurs gradually and only in an ionic environment once the microspheres are delivered to the targeted tumor, which further underlined the pharmacokinetic difference between this targeted therapy and cTACE. Beads with larger diameters (700 to 900 µm) seem to release doxorubicin more slowly than the smaller beads.11 The continuous release of doxorubicin from LC Beads to the tumor tissue has been demonstrated in several animal experiments.6,12 Furthermore, histopathologic analysis described the high efficiency of DEB-mediated drug delivery and release to the tumor tissue, causing local coagulative necrosis and an inflammatory fibrotic tissue.13
The other types of microspheres (QuadraSphere/HepaSphere microspheres) consist of superabsorbent polymer (SAP), a nonbiodegradable material which is hydrophilic and highly absorbent. These spheres have the ability to absorb fluids up to 64 times their dry state volume and thus to expand their volume to a size of up to 800 µm. These microspheres can be loaded with doxorubicin, epirubicin, and cisplatin for drug delivery.14 In summary, it is safe to say that the technical evidence clearly is in favor of the use of DEBs, but its general acceptance worldwide as a possible replacement for cTACE has not yet been officially endorsed.
PATIENT SELECTION/CONTRAINDICATIONS
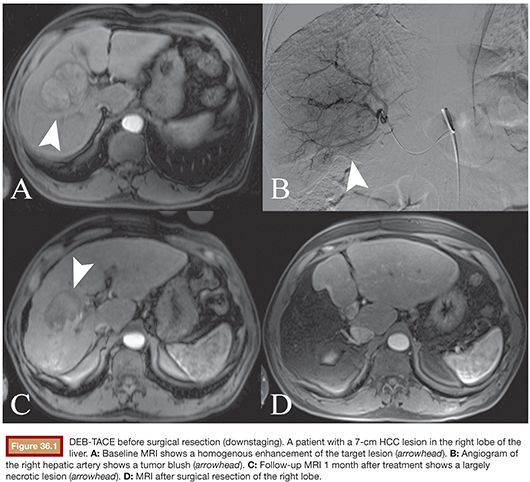
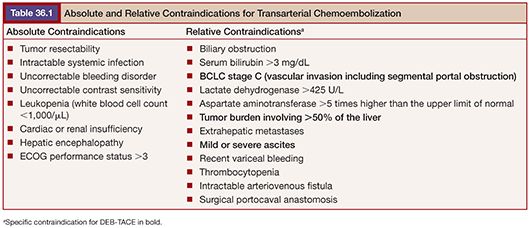
To date, DEB-TACE has been used in various clinical settings for palliative treatment of patients with unresectable primary liver cancer. Furthermore, it has been successfully used as an adjunctive option before tumor resection or as a bridge to orthotopic liver transplantation15 (Fig. 36.1). In general, DEB-TACE can be indicated in patients with liver-dominant, unresectable HCC. However, the selection of patients must be based on the presence of adequate liver function, sufficient performance status (Karnofsky index, Eastern Cooperative Oncology Group performance status [ECOG]), and the absence of extrahepatic spread.16 Table 36.1 summarizes the most important and universally valid contraindications for TACE procedures, which includes DEB-TACE. Because DEB-TACE is still relatively new and lacks sufficient clinical data, the list of exclusion criteria is more extensive as compared to cTACE.
TECHNIQUE AND RECOMMENDATIONS
All TACE procedures share a common approach. Following, we describe the Johns Hopkins Hospital technique for DEB-TACE using LC Bead microspheres, which is in agreement with the results of a consensus meeting held during the European Conference on Interventional Oncology17 in 2012:
1. Evaluation: Initially, multiple diagnostic angiographic steps are performed to define the hepatic arterial anatomy, assess the blood supply to the tumor, and determine portal venous patency.
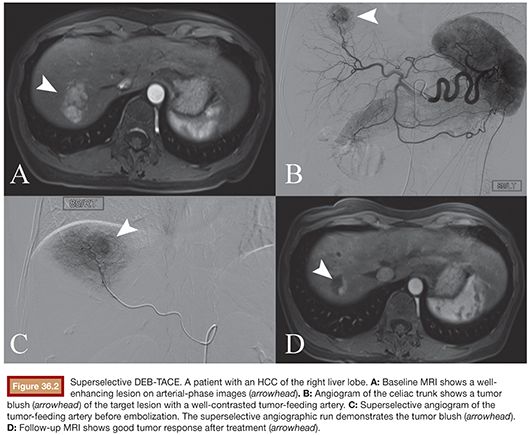
2. Catheter positioning: According to the latest recommendations, whenever possible, a 2-Fr microcatheter is placed for selective (fourth-order branch) to superselective (segmental or even subsegmental) injection (Fig. 36.2). DEBs (LC Beads), which have been preloaded with 100 mg of doxorubicin in the central oncology pharmacy, are suspended in equal volume of nonionic contrast medium (1:1) and left for 2 to 3 minutes to become homogeneously distributed within the syringe.
3. Injection: Up to 4 mL of the DEBs (loaded with 25 to 37.5 mg of doxorubicin per milliliter of beads) are administered by alternating injections of aliquots of the beads and contrast until complete delivery or when the blood flow of the feeding artery slowed down. The injection of DEBs should be performed slowly under fluoroscopic guidance to ensure that there is no reflux. Rotation of the syringe is also recommended to prevent sedimentation of the material. No injection of iodized oil (Lipiodol or Ethiodol) is required.
4. Embolization end point: Substantial arterial flow reduction to the tumor is the technical end point, whereas complete occlusion is avoided. This is essential to maintain the arterial pathway for retreatment. Repeated DEB-TACE sessions are recommended, and in our institution, an average of two treatments per target lesion is usually necessary to complete a cycle.
POTENTIAL COMPLICATIONS
Various clinical trials confirmed the overall safety of TACE procedures. The reported systemic adverse effects include nausea, vomiting, bone marrow aplasia, renal failure, and, potentially, cardiac toxicity. The self-limiting postembolization syndrome (PES) includes nausea, vomiting, fever, right upper quadrant pain, and increased white blood cell count. These symptoms reflect the effects of tumor necrosis, acute cytokine release, and systemic exposure to chemotherapeutic agents.18,19 The use of DEBs has contributed to a significant decrease in systemic toxicities after TACE, and the PES following DEB-TACE is not as intense as following cTACE. The systemic side effects of DEB-TACE using doxorubicin range from alopecia and skin discoloration to mucositis and bone marrow suppression. Cholecystitis and liver abscess may occur less frequently. Periprocedural mortality is seen in less than 1% of cases.20
In one of the few studies of its kind, a prospective, randomized single-center phase II trial from Belgium thoroughly investigated plasma peak concentrations of doxorubicin after DEB-TACE using SAP microspheres in comparison with cTACE. As a result, patients treated with SAP microspheres showed significantly lower plasma peak concentrations and less systemic toxicities.21 These results were confirmed for LC Beads by a multicenter, randomized, prospective phase II trial, which compared the safety and toxicity of DEB-TACE to that of cTACE in HCC patients. Here, the DEB-TACE procedure showed a significantly better toxicity profile than cTACE. This was true in terms of the overall frequency of treatment-related adverse effects as well as the toxicity grades and the severity of the adverse events. Analysis of true toxicity incidence revealed the presence of significant events only in 11.8% of patients treated with DEB-TACE versus 25.9% of patients treated with cTACE. More importantly, major liver toxicities were also lower in DEB-TACE as compared to cTACE.3 In conclusion, both studies confirmed that DEB-TACE is both safe and generally better tolerated by patients than cTACE.
OUTCOMES
The advent of DEBs has led to a number of trials using this new drug delivery system for TACE procedures. The first FDA-sanctioned experience with DEB-TACE in the United States was designed as a prospective phase II pilot study with the goal to evaluate safety, efficacy, as well as progression-free survival and overall survival in 20 mostly cirrhotic (80%) patients with unresectable HCC. Most of the treated patients were staged as Child-Pugh A (75%), whereas 12 patients (60%) were classified as Barcelona Clinic Liver Cancer (BCLC) stage C. According to the European Association for the Study of the Liver (EASL) criteria, 64% of the patients were classified as responders and 30% achieved complete response after an overall 34 sessions. As for safety, only modest toxicities were reported in all patients, once again confirming the advantages of DEB-TACE over cTACE. After 6 months, only 1 patient showed progressive disease according to the Response Evaluation Criteria In Solid Tumors (RECIST). The median overall survival of 26 months in most advanced patients (BCLC stage C) confirmed the potential of DEB-TACE as a better alternative than cTACE.4
The important PRECISION V study included 15 international centers and enrolled a large number of patients for a prospective, randomized phase II trial. The goal of this trial was to directly compare the safety and efficacy of cTACE to those of DEB-TACE. A total of 212 patients were randomized 1:1 and 201 patients received treatment according to standardized protocols. The two treatment groups were stratified according to ECOG performance status and the Child-Pugh class. As a result, patients in the DEB-TACE group showed better imaging response to treatment according to EASL criteria than those in the cTACE group. Although at the 6-month follow-up time point (the end point of the study) the complete response rate was nearly identical between the two groups (26.6% and 22.2% complete response in the DEB-TACE and cTACE groups, respectively), the rate of progressive disease was significantly lower in the DEB-TACE than in the cTACE group (32.3% vs. 40.7%).3 Of note, this trial was not designed to compare overall survival between the two groups of patients, which is required to fully confirm the potential of DEB-TACE as a true alternative to cTACE.
The lack of survival studies in DEB-TACE was addressed by one of the longest follow-up studies available to date with DEB-TACE. Confirming the great promise of DEB-TACE, a prospective multicenter study from Greece included 173 unresectable HCC patients to assess long-term clinical outcome. Results revealed a 5-year survival of 29.4% and 12.8% for Child-Pugh class A and B, respectively.22 These latest data on DEB-TACE clearly show the potential of that therapy. In an attempt to compare overall survival in patients treated with DEB-TACE with outcomes after cTACE, a retrospective trial included a total of 71 patients with unresectable HCC, 63% of whom received DEB-TACE and 37% cTACE treatment. As a result, median overall survival of patients treated with DEB-TACE was 610 days versus 284 days in the cTACE group, suggesting a survival advantage of DEB-TACE over cTACE. However, the results of this study must be interpreted with great caution because of the highly inhomogeneous groups and a lack of strict inclusion criteria. In summary, prospective randomized trials will be needed to deliver definitive data on patient survival before fully including DEB-TACE in the official guidelines. As of today, it is safe to say that DEB-TACE is better tolerated than cTACE with a clearly better toxicity profile.
DEB-TACE IN COMBINATION WITH SYSTEMIC TREATMENT
The main antitumor effects of both cTACE and DEB-TACE are due to a combination of ischemia and direct chemotherapy-induced cytotoxicity. The capability of chemoembolization to cause massive tumor destruction has been well demonstrated in multiple radiopathologic correlation studies23; however, tumor recurrence is frequently encountered. It has been postulated that the reason for tumor recurrence is the stimulation of neoangiogenic pathways that are significantly upregulated within 36 hours of TACE. The molecular mechanism behind this reaction is likely triggered by hypoxia within the tumor tissue caused by embolization of the tumor-feeding arteries. Indeed, molecular surrogate markers of tumor hypoxia including hypoxia-inducible factor-1 alpha (HIF-1α) as well as vascular endothelial growth factor (VEGF) are upregulated after TACE procedures, suggesting direct stimulation of angiogenesis.24,25 As a result of these findings, inhibiting the angiogenic pathway during treatment with TACE seems to be logical. Multiple approaches to target this mechanism have been discussed. One such approach uses the drug sorafenib, a multikinase inhibitor with strong antiangiogenic properties, in combination with DEB-TACE. In this way, the proangiogenic changes induced by embolization within the tumor would possibly be counterbalanced by sorafenib, which would be used as a “partner” for TACE.26 Of note, sorafenib has shown to significantly prolong patient survival over placebo in a randomized controlled trial that led to the FDA approval of the drug for patients with HCC, making sorafenib the very first systemically applicable drug to improve survival in patients with HCC.27 Given this scientific rationale and the demonstrated impact of sorafenib on patient survival, several prospective clinical trials have been designed (and many are still underway) to assess the potential of this truly unique combination. The following paragraphs will summarize the results of the most important trials.
In one of the first FDA-sanctioned experiences with the combination of DEB-TACE and sorafenib in the United States, a single-center prospective phase II trial was conducted to evaluate the safety and efficacy of concurrent sorafenib and DEB-TACE therapy. A total of 35 individuals with unresectable HCC were enrolled. Major inclusion criteria were an ECOG performance status of 0 to 1, Child-Pugh liver function up to B7, and patients with segmental portal vein thrombosis (BCLC stage C). Patients were treated on a 6-week cycle regimen, in which one cycle consisted of 400 mg sorafenib twice daily, initiated 7 days before the first DEB-TACE. The 35 patients were treated with a total of 128 cycles of therapy. All patients received DEB-TACE and the mean dose of doxorubicin decreased over time: cycle one, 75 mg; two, 60 mg; three, 49 mg. Safety and toxicity were defined as the primary end points of this study, whereas efficacy was the secondary end point. All patients experienced at least one treatment-related toxicity during cycle one. However, most toxicities were minor and only 17% of all toxicities were grade 3 to 4. According to EASL criteria, 58% of the patients showed objective tumor response and the disease control rate was 100%, with no patient showing progression. This study was the first to confirm the safety profile of the DEB-TACE/sorafenib combination.26 It must be noted that sorafenib was given continuously through the planned DEB-TACE treatments, making the treatment protocol of this trial truly unique. Most other trials used sorafenib either in an interrupted or sequential manner with TACE. As for the secondary end point of this trial, the published data also demonstrated promising survival outcomes and follow-up studies will answer more questions.
An international attempt to investigate the role of the combination of DEB-TACE with sorafenib was the first multicenter phase II randomized, double-blind, placebo-controlled clinical trial (“SPACE” = Sorafenib or Placebo in Combination with DEB-TACE for Intermediate-Stage HCC). This comprehensive trial, which enrolled patients across 85 centers in Europe, North America, and Asia, was recently presented (not yet published). Here, a total of 307 eligible patients were randomized to receive either sorafenib (n = 154) or placebo (n = 153) in combination with DEB-TACE. The dosage was similar to the trial described earlier and patients received a dose of 400 mg sorafenib twice daily or a matching placebo continuously on cycle duration of 4 weeks. All patients received DEB-TACE within the first week after the first dose of sorafenib/placebo and subsequently on day 1 of cycle 3, 7, and 13, respectively. The primary end points of this study were efficacy (specifically, the time to tumor progression [TTP]) and safety. The secondary end points were overall survival, time to vascular invasion, as well as other surrogate markers of tumor progression. As a result, median TTP was 169 days in the sorafenib group and 166 days in the placebo group, itself a rather unexpectedly moderate result. When further stratified, TTP at the 25th and 75th percentile was 112 days/88 days and 285 days/224 days in the sorafenib and placebo groups, respectively. Interestingly, the hazard ratios were low, favoring the combination treatment over the placebo arm. However, the overall preliminary results were somewhat disappointing as no statistically significant benefits regarding overall survival and TTP were shown.28
Multiple further trials are underway combining DEB-TACE as well as cTACE with sorafenib. It might be very well possible that the answer about the combination of sorafenib and TACE will be provided by the only phase III trial that is already underway in the United States. This randomized, double-blind, controlled multicenter trial which was proposed by one of the cooperative oncology groups (E1208) will compare the outcomes of DEB-TACE with or without sorafenib in HCC patients with or without vascular invasion (NCT01004978). The results of this trial should be available at the end of 2014.
TIPS AND TRICKS
• Preference is to use a glide Simmons 1 (Terumo, Tokyo, Japan) for selective catheterization of the superior mesenteric artery (SMA) and celiac axis. In most instances, there is no need for a flush aortogram. One can proceed directly to the visceral angiograms.
• Do the SMA run first to identify possible arterial supply to the liver from the SMA and confirm portal vein patency.
• It is critically important to recognize the arterial anatomy and make sure selective catheter placement is performed distal to potentially “dangerous” vessels such as omental or peritoneal branches, right gastric artery, and other small gastric or periduodenal arteries. Inadvertent administration of beads to any of these vessels may cause significant complications, hence the extra care in identifying and avoiding them.
• For centrally located liver tumors, evaluate bilateral blood supply by performing contrast runs from both hepatic arteries.
• Be aware that tumors identified on cross-sectional imaging (magnetic resonance imaging [MRI] or computed tomography [CT]) as being in the right or left lobe may not be supplied by the corresponding arteries; for example, a tumor located in segment 8 may actually be supplied by the segment 4 branch.
• Always perform a pretreatment dual-phase cone-beam CT as selective microcatheter placement in close proximity to the tumor is essential.
• If available, try to use a dedicated three-dimensional analysis software to help identify the appropriate tumor-feeding arteries and create a road map.
• While attempting to place the microcatheter superselectively by manipulating the guidewire, always make sure to carefully watch the Simmons catheter as well so it does not get displaced back into the aorta (Fig. 36.2C).
• When injecting the DEBs, make sure to suspend the beads frequently to prevent the beads from settling down. Preference is to use a 10-mL syringe and inject the DEBs slowly. The beads should be mixed with contrast in a 1:3–4 ratio for greater visibility once the beads leave the microcatheter. The injection should also be done slowly to ensure forward flow and prevent reflux of the beads along the catheter shaft.
• Always avoid stasis of the main artery to allow retreatment if it is necessary. The end point of the embolization should be 2–5 heartbeats to clear the contrast column or administration of the entire dose. Never add additional bland particles.
• Perform a cone-beam CT scan immediately after DEB-TACE so contrast retention within the tumor(s) can be readily identified. This scan must be done before hemostasis of the common femoral artery is obtained.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree