Chapter 19 Chemotherapy and hormones
Introduction
Principles of cytotoxic therapy
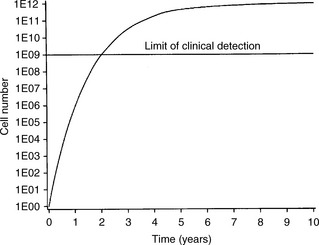
To understand the rationale of cytotoxic chemotherapy it is important to recognize the features of tumour growth. The asymmetric sigmoidal growth curve, the ‘Gompertzian growth curve’ (Figure 19.1) describes the natural history of tumour growth. By the time that a tumour is clinically detectable, the majority of its growth has already occurred. In the early exponential phase of growth, the rates of tumour cell growth and tumour cell loss are proportional to the tumour cell burden at any point. Since most anticancer agents are more toxic to proliferating cells and most tumours are in a relatively slow phase of growth when diagnosed (i.e. they lie high and towards the plateau of the Gompertzian growth curve), it explains the limited effectiveness of chemotherapy for many cancers. The reason for tumour cytoreduction (e.g. by surgery) before chemotherapy is to bring the tumour to a lower point on the growth curve when the growth fraction of the tumour rises. The concept of moving the tumour down the Gompertzian curve underpins the rationale of adjuvant chemotherapy.
Drug resistance
1. changes in the cell membrane impeding drug transport (e.g. of methotrexate)
2. DNA repair of drug-induced lesions (e.g. caused by cisplatin)
3. utilization of alternative metabolic pathways (e.g. 5-fluorouracil)
4. increased production of a target enzyme (e.g. dihydrofolate reductase binding to methotrexate)
5. modification of the target enzyme, enabling it to recognize the difference between true and false metabolites (e.g. 6-mercaptopurine).
Side effects of chemotherapy
The main normal tissues damaged by cytotoxic therapy are those with rapidly dividing cell populations: the bone marrow, the gastrointestinal epithelium, the hair and the germ cells of the testis. By contrast, there is little effect on non-proliferating tissues, such as skeletal muscle and nervous tissue. The most common side effects of individual cytotoxic drugs are shown in Table 19.1. The most dramatic improvement in the management of side effects was with the development of the 5-hydroxytryptamine antagonist antiemetics. More recently the Neurokinin-1 antagonist aprepitant has improved the emesis of patients undergoing cisplatin based chemotherapy. Typical antiemetic regimens are shown in Table 19.2. Other drugs that have improved drug delivery have been the recombinant growth factors for red (erythropoietin) and white cells (human granulocyte colony-stimulating factor (G-CSF)). These agents stimulate the release of progenitor cells from the bone marrow and are useful in the treatment and prevention of anaemia and neutropenia.