Circulatory Disturbances
John H. Juhl
Janet E. Kuhlman
J. H. Juhl and J. E. Kuhlman: Department of Radiology, University of Wisconsin Medical School, Madison, Wisconsin 53792-3252.
PULMONARY EDEMA
Pulmonary edema is the term used to indicate an abnormal accumulation of fluid in the extravascular pulmonary tissues.25 There is a constant flow of fluid and proteins from the microvascular spaces (arterioles, capillaries, and venules) in the lung into the interstitial space.32 The interstitial space includes the alveolar wall interstitium and the interlobular, perivascular, peribronchial, and subpleural connective tissue spaces.
The flow can increase from an estimated 20 mL/hour to about 8 to 10 times that amount in the normal person without causing pulmonary edema or an appreciable increase in extravascular lung fluid. Respiratory pumping action appears to be an important factor in lymphatic flow. Direction of flow is determined by valves in the lymphatics.
The pulmonary lymphatic vessels begin in loose connective tissue spaces proximal to but not in the alveoli. There are five groups of lymphatics in the lungs36: (1) Pleural—common over the lower lobes (95% to 100%) and uncommon over the upper and middle lobes (14% to 31%). The flow is for a variable distance on the surface and then to the hilum via the interstitial spaces of the interlobular septa. (2) Interlobular—arise at the periphery of the acinus (not in the alveolar walls) and extend in the septa to join with the veins, where they become (3) the perivenous group, which extend to the hilum. (4) Peribronchial—arise at the junctions of the alveolar septa and accompany the pulmonary arteries and bronchi to the hilum. (5) Anastomotic—run in the deep interlobular septa.
The lymphatics in the juxta-alveolar spaces have a poorly developed discontinuous basement membrane and endothelium with partially open cell junctions so that fluid can be cleared rapidly. The pulmonary lymphatics carry the excess microvascular filtrate into the systemic venous system. When the capacity of the lymphatics is exceeded, edema results. Endothelial cells form a continuous lining of the alveolar capillaries and the remainder of the pulmonary vascular system. Connective tissue fibers weave through the interstitium in such a way that one half of two thirds of the alveolar capillary walls have an interstitial space; elsewhere, the capillary endothelium and the alveolar epithelium are fused. The endothelial cell junctions are not as tight as those in alveolar epithelium and are therefore more permeable. Fluid and sizeable protein molecules can escape through endothelial and epithelial junctions without causing permanent damage, which probably accounts for the rapid resolution of pulmonary edema in some patients after the inciting cause is removed.
The major causes of pulmonary edema and conditions associated with them are as follows:
Hemodynamic pulmonary edema (elevated capillary hydrostatic pressure—the most common form)
Left heart failure
Mitral valvular disease
Left atrial tumor (myxoma)
Pulmonary venous obstructiona
Mediastinal fibrosis
Mediastinal tumor
Veno-occlusive disease
Neurogenic (hemodynamic in some cases, if not all)
Permeability pulmonary edema
Narcotic overdose?
Salicylate poisoning
Smoke inhalation
Inhalation of chemical fumes including
High altitude
Near-drowning
Rapid re-expansion of lung (e.g., thoracentesis, removal of large pneumothorax)b
Snake venom and other circulating toxins
Aspirated hydrocarbons
Post-traumatic fat embolism
Irradiation
Epiglottitis (acute upper airway obstruction)?
Combined hemodynamic and permeability edema
Shock lung, including septic shock
Adult respiratory distress syndrome (ARDS)—a variety of causes
Intravenous iodinated contrast media?
Narcotic overdose?
Renal disease edema?—combination of hypervolemia, hypoproteinemia and increased hydrostatic pressure; fluid overload is similar to renal disease without hypoproteinemia
Lymphatic obstruction edema—mechanical, anatomic, or neurogenic
Edema of unknown origin
The pathophysiology of several of these causes is still controversial, and the list is incomplete, since new conditions causing pulmonary edema are reported frequently.
Decompression sickness in divers and tunnel workers is recognized as a cause of noncardiogenic pulmonary edema.42 It occurs within 6 hours of rapid decompression. Because it can be life-threatening, hyperbaric chamber recompression is recommended. The pathogenesis of edema in these cases is complex because nitrogen bubbles may obstruct and produce ischemia, leading to altered permeability distally and an increase in hydrostatic pressures proximally, resulting in overperfusion. A release of vasoactive substances has also been considered to be part of the process by some.
Clinical symptoms of pulmonary edema are varied and depend on the associated disease or injury. When the edema is extensive and acute there is usually severe respiratory distress, but when the onset is insidious, particularly in uremia, there may be very few respiratory symptoms. There is a notable discrepancy between roentgen and physical findings in chronic pulmonary edema and in some patients with acute or subacute interstitial edema. Therefore, the radiographic examination of the chest is very important. Two major roentgen patterns of edema are observed, depending on the site of the transudate—namely, alveolar and interstitial. Fluid enters the interstitial spaces from the pulmonary capillaries to produce interstitial edema, and, as the amount of extravascular fluid increases, a point is reached at which it escapes into the alveoli. At first, the amount of alveolar filling may be minimal, but as the amount of fluid increases, the edema progresses and can become very extensive.
INTERSTITIAL EDEMA
As indicated, interstitial edema precedes alveolar edema; therefore, it is necessary to be able to recognize the interstitial fluid in order to determine the presence of congestive heart failure or other causes of edema early in the course of the disease.
There are several signs of interstitial edema which are reliable as a group, particularly when correlated with the clinical findings. They are:
Perivascular blurring or cuffing, in which the margins of the vessels become indistinct and widened in the parahilar area extending out to involve vessels in the lung parenchyma.
Peribronchial blurring or cuffing with loss of clear definition of the outer bronchial wall as seen in cross-section on the roentgenogram.
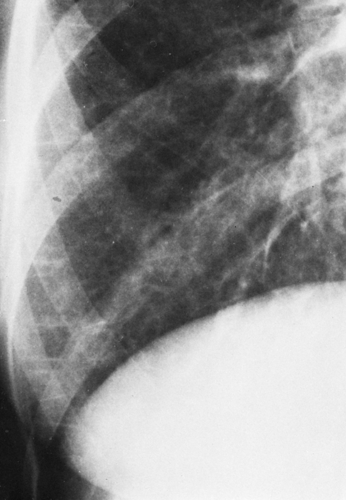
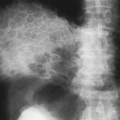
“Hilar haze”—a loss of definition of large central pulmonary vessels with a slight general increase in opacity. This is very likely caused by interstitial edema anterior and posterior to the hilum, because the central vessels do not have perivascular interstitium until they enter the lung. This is probably the phase in which the alveolar wall interstitium contains excess fluid; it is very difficult to evaluate and is an accessory sign at best (see Fig. 28-1). It is often best appreciated when an earlier film is available for comparison; when a film is obtained after successful treatment of the edema, it can be recognized in retrospect.
Appearance of septal lines
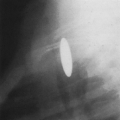
Kerley B lines are dense, horizontal lines that measure about 1.5 to 2.0 cm in length. They are best seen in the lower lung on oblique projections on films of good quality. They represent secondary interlobular septa thickened by fluid (Figs. 28-1 and 28-2).
Kerley A lines are longer and range in length from 5 to 10 cm. They tend to be straight or slightly curved and extend from the hila or parahilar area toward the periphery. They are seen in the upper lobes and tend to appear in acute interstitial edema. They represent fluid in the secondary interlobular septa, chiefly in the upper lobes (see Fig. 28-1).
A diffuse, reticular pattern may be observed in association with other findings noted in the signs already described. This is difficult to assess but probably represents interstitial fluid in these patients with edema. A similar pattern may be seen in patients with widespread interstitial fibrosis, but Kerley A lines usually are not present, so the differentiation usually is not difficult.
In chronic congestive failure, interstitial edema may resemble chronic interstitial thickening associated with pulmonary disease. In some cases, the true nature of the process can be diagnosed with certainty only after treatment of the cardiac condition brings about regression. Interstitial edema
often occurs in combination with the alveolar type (Fig. 28-3).
Interstitial edema precedes alveolar edema in these instances, but often the onset is very rapid, with massive alveolar edema overshadowing all subtle signs.
Subpleural edema may be observed best adjacent to the minor fissure on the right, but it may also be observed along the major fissures in lateral projections. Peripherally there may be enough edema fluid to simulate pleural thickening.
The fluid accumulates first in peribronchial, perivascular, and interlobular interstitial spaces to produce the findings as noted. Edema (thickening) of the alveolar wall probably does not occur until later, after the other interstitial spaces are filled. Then alveolar flooding may occur if fluid continues to accumulate in the lungs.
ALVEOLAR EDEMA
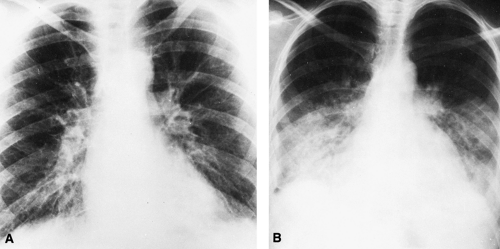
The classic roentgen findings of alveolar pulmonary edema are those of bilateral opacities that extend outward in a fan-shaped manner from the hilum on both sides (Figs. 28-4, 28-5, 28-6, 28-7, and 28-8). The peripheral lungs are relatively clear. This includes the bases as well as the apices except in congestive failure, in which basal congestive changes and edema produce changes there. Because interstitial edema precedes the alveolar form, some or all of the signs of interstitial edema may be observed. However, the alveolar edema may be so extensive that other signs are obscured. When the edema is moderate, the opacity is somewhat patchy and mottled, but it may become quite homogeneous as the amount increases (Fig. 28-4). In the latter instance, the fluid-filled
alveoli surrounding the bronchi produce contrast with the air-containing bronchi and, as a result, the bronchi are visible as linear radiolucent spaces (air bronchogram) traversing the opaque edematous area (Figs. 28-5). The opacity is often bilaterally symmetrical, or nearly so. There are many exceptions to this rule, and a number of cases have been reported in which the edema was unilateral.
alveoli surrounding the bronchi produce contrast with the air-containing bronchi and, as a result, the bronchi are visible as linear radiolucent spaces (air bronchogram) traversing the opaque edematous area (Figs. 28-5). The opacity is often bilaterally symmetrical, or nearly so. There are many exceptions to this rule, and a number of cases have been reported in which the edema was unilateral.
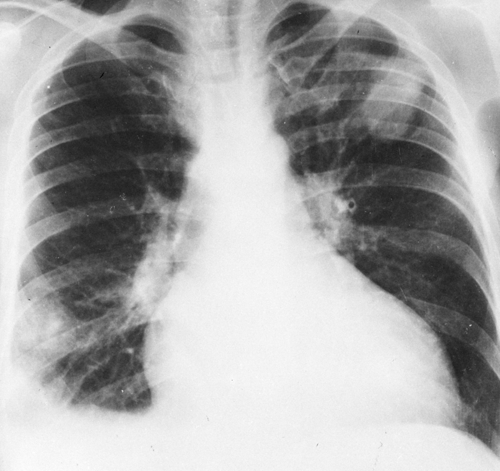
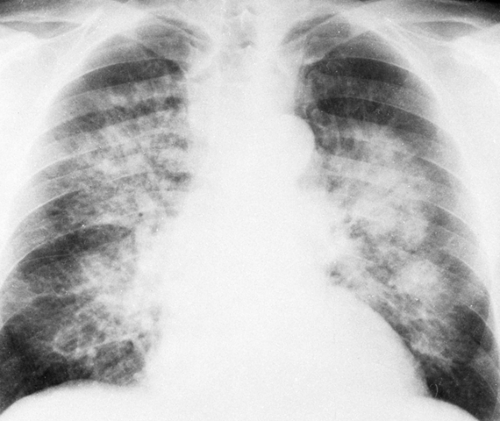 FIG. 28-4. Pulmonary edema. This is a rather typical example of alveolar edema, with a fan-shaped distribution in the parahilar and middle zones of the lungs. The patient had chronic renal failure. |
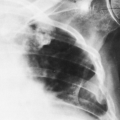
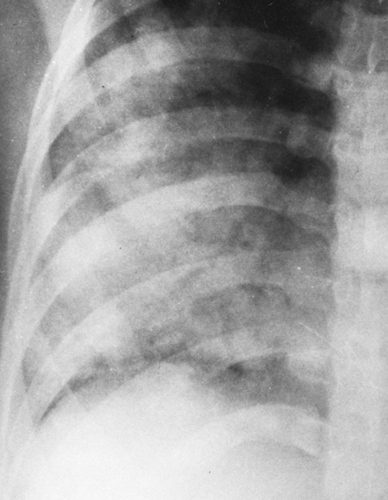 FIG. 28-5. Alveolar pulmonary edema. Closeup view of the right lower lung shows air-filled bronchi (air bronchogram) in the otherwise dense, fluid-filled lung. |
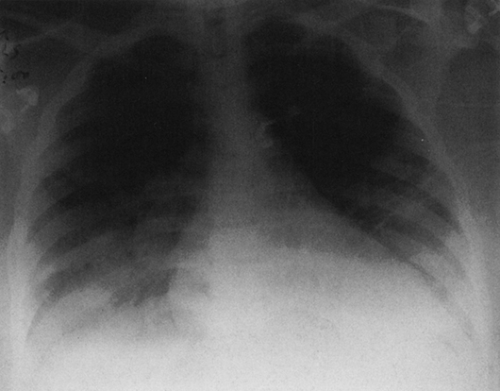
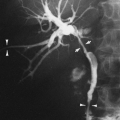
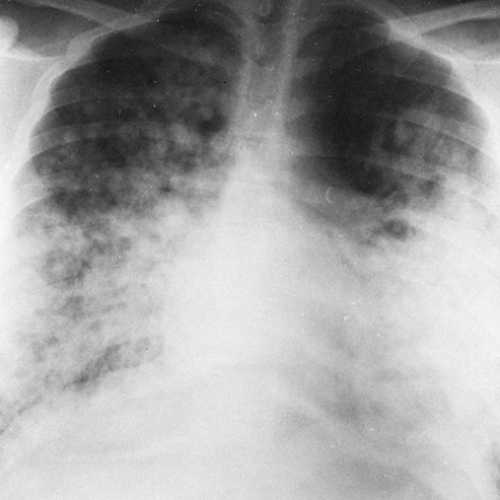 FIG. 28-6. Alveolar edema. The rounded, poorly defined opacities noted best in the upper lung and in the right central and lower lung represent the acinar type of fluid distribution. |
Serial films often show rapid changes in the amount and distribution of the edema from day to day. When pulmonary edema is early and minor, it may produce scattered, localized opacities that simulate nodular disease (Fig. 28-6). The “nodules” are poorly defined, however, and probably represent edema fluid filling the acini (the portions of lung distal to terminal bronchioles). Bizarre forms may also be present, with large, rather rounded areas of increased density that may simulate tumor. As a general rule, the alveolar opacity is hazy and poorly defined, so there is no difficulty in making the diagnosis. Pleural effusion is commonly associated with edema, particularly in congestive heart failure and in uremia.
Numerous reports of unusual and asymmetrical distribution of edema have led to some experimental work and much speculation as to the factors involved in the distribution of edema fluid.10 Gravity is undoubtedly a factor in many cases, but lateral views of patients who have been supine sometimes show the fluid to be in anterior or lingular segments or in the middle lobe. Lack of peripheral edema is probably related to better peripheral drainage of lymphatics and to the increased respiratory motion of the peripheral lung acting to “pump” the fluid out. The relative lack of compliance in the central lung and in lung that has been previously diseased may also be involved. Defective lymphatic drainage favors accumulation of fluid in areas of pulmonary disease; this has resulted in scarring or decrease in compliance.
Gravity definitely plays a part in many patients with unilateral pulmonary edema. Other factors include differences in perfusion and in microvascular pressures in different areas. A gravitational shift test has been devised and studied to detect edema and to differentiate it from other causes of lung opacity.41 The patient is kept in a supine or semierect position for 2 hours before a baseline bedside radiograph is obtained. Then the hemithorax with the lesser density is placed in a dependent position and the patient is kept in this lateral decubitus position for between 2 and 3 hours. A second bedside radiograph is then obtained. In this study there was a recognizable shift of fluid opacity to the dependent side in 85% of patients with edema and in 86% of patients with edema and inflammatory disease. There was no shift in 78% of patients with inflammatory disease only. Therefore, this test may be of help in differentiating edema from other pulmonary conditions in some cases.
There are no constantly reliable changes that identify the cause, but in edema secondary to cardiac failure the observations
of cardiac enlargement, pulmonary vascular redistribution to the upper lobes, basal edema, and pleural effusion are strong indications that the edema is the result of heart disease. Exceptions include the absence of cardiac enlargement in some patients with pulmonary edema secondary to acute coronary thrombosis and the absence of basal congestive changes in patients with edema secondary to acute left ventricular failure. As a general rule, pulmonary edema caused by uremia (azotemic edema) produces the classic central perihilar opacity of the lungs, with or without evidence of cardiac enlargement and balanced pulmonary vascularity.
of cardiac enlargement, pulmonary vascular redistribution to the upper lobes, basal edema, and pleural effusion are strong indications that the edema is the result of heart disease. Exceptions include the absence of cardiac enlargement in some patients with pulmonary edema secondary to acute coronary thrombosis and the absence of basal congestive changes in patients with edema secondary to acute left ventricular failure. As a general rule, pulmonary edema caused by uremia (azotemic edema) produces the classic central perihilar opacity of the lungs, with or without evidence of cardiac enlargement and balanced pulmonary vascularity.
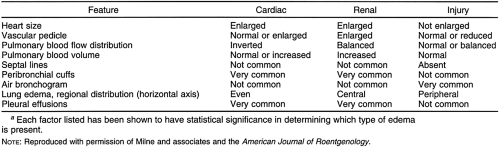
Pulmonary edema (injury edema, permeability edema) caused by inhalation of irritant gases tends to be somewhat more widespread than the other types and results in a mottled and patchy appearance extending farther to the periphery with slightly less central involvement than that seen in uremia; it also tends to be more basal. Roentgen distribution is not characteristic, however, and the history is of great importance in arriving at the diagnosis in these patients. Milne and coworkers studied the etiology of pulmonary edema and showed that the cause can often be determined on plain chest radiographs.20,21 A remarkable degree of accuracy (91%) was obtained in differentiating capillary permeability edema from other varieties. The lowest accuracy (81%) was in distinguishing cardiac failure from renal failure, and the overall accuracy ranged from 86% to 89%. The factors listed in Table 28-1 were evaluated in this study.
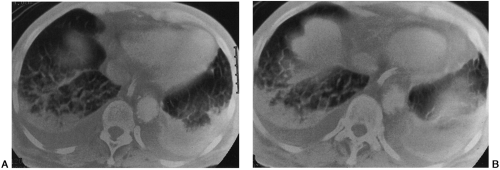
Computed tomographic (CT) findings of hydrostatic pulmonary edema have been recognized. They include engorgement and enlargement of the pulmonary vessels; smooth, regular thickening of the interlobular septa as a result of interstitial edema; areas of ground-glass attenuation caused by alveolar edema; and bilateral pleural effusions22 (Fig. 28-9).
PULMONARY THROMBOEMBOLISM
Pulmonary embolism with or without infarction is a more common lesion than is generally realized. Because the majority of thromboembolic episodes cause no symptoms, they are not recognized. In addition to its occurrence as a postoperative
complication and in patients with cardiac disease, pulmonary embolism occurs in a number of other conditions, including immobilization for any reason (e.g., sitting or standing for long periods, leg casts, stroke, pregnancy, oral contraceptives, varicose veins). The most common sources of pulmonary emboli are thrombi in the deep veins of the thigh, the pelvis, and, to a lesser extent, the calf. If the embolus is very large and occludes almost the entire pulmonary arterial tree, death can occur very quickly. If the embolus is somewhat smaller it may or may not produce infarction, but it often causes immediate symptoms of chest pain and dyspnea. There may be no symptoms or signs with small emboli.8
complication and in patients with cardiac disease, pulmonary embolism occurs in a number of other conditions, including immobilization for any reason (e.g., sitting or standing for long periods, leg casts, stroke, pregnancy, oral contraceptives, varicose veins). The most common sources of pulmonary emboli are thrombi in the deep veins of the thigh, the pelvis, and, to a lesser extent, the calf. If the embolus is very large and occludes almost the entire pulmonary arterial tree, death can occur very quickly. If the embolus is somewhat smaller it may or may not produce infarction, but it often causes immediate symptoms of chest pain and dyspnea. There may be no symptoms or signs with small emboli.8
In addition to thrombi, a number of other materials can act as emboli. Air embolism may follow trauma, surgical procedures, intravenous procedures of all types, thoracentesis, percutaneous lung biopsy, or any manipulation with the potential of exposing a vein to air. The air is not usually demonstrated radiographically; it disappears very quickly if the patient survives. If air embolism is not immediately lethal, air may be observed in the pulmonary artery; air was observed in the heart of a patient who subsequently died.15 Pulmonary edema may result when the patient survives, because venous air embolism affects the lungs. If air embolism is suspected, the patient should be placed in the left lateral decubitus position, to keep the air in the right atrium, if possible, until it is absorbed.
Arterial air embolism results when air enters the pulmonary venous system; it affects the heart and brain. If it causes sudden death, the air can usually be demonstrated on postmortem films. Opaque contrast materials used in lymphangiography, hysterosalpingography, and myelography occasionally enter the bloodstream and have been demonstrated in the lungs after these procedures. A few cases of barium in the pulmonary vessels after barium enema have been reported in which fatalities have occurred during the examination. Metallic mercury injected intravenously has been reported in a few instances. Dense, small, globular, and branching linear opacities may then be demonstrated on the chest radiograph.
PULMONARY INFARCTION
The incidence of infarction varies in different groups of patients with pulmonary thromboembolism. In patients with chronic heart disease and congestive failure, it approaches 100%, but in young, healthy persons, infarct with necrosis is rare unless there are complicating factors such as severe trauma. In the elderly and in chronically ill patients who are bedridden, the incidence of infarct is in the range of 60% to 70% of those who have pulmonary emboli. The roentgenographic diagnosis is often difficult to make. Often no abnormalities are present on the chest film.9 The major error is usually failure to suspect infarction as the cause for abnormal roentgen findings in the chest. The roentgen signs are (1) elevation of the hemidiaphragm on the involved side, indicating decrease in lung volume; (2) unilateral pleural effusion, usually small; (3) pulmonary parenchymal consolidation; (4) atelectasis; and (5) linear shadows. An infarct must be differentiated from pneumonia, edema, and atelectasis as well as other local conditions, including infected cysts and abscesses. The lower lobes are most frequently involved, but the lesion can occur in any lobe. Elevation of a hemidiaphragm or a small, unilateral pleural effusion, or both, may be the earliest signs of infarction. At times the shadow of the infarct may not be visible and the effusion is the only sign. It takes 10 to 24 hours for an infarct to evolve to the point where it is visible roentgenographically. This is probably the hazy, poorly defined, edematous lesion that requires an additional 2 to 4 days and sometimes 1 week to form a well-defined, complete infarct.
Infarction of a single secondary pulmonary lobule can occur, and, because not all lobules have a pleural surface, it follows that not all infarcts have a pleural surface. Infarcts are usually aggregates of involved secondary lobules, however, so the lesion usually extends to a pleural surface. Because this may be the interlobar pleura, its shadow is not necessarily peripheral as visualized in the frontal projection. The shape of the infarct depends on the location. The visceral pleura usually forms one side of the lesion, and often two or three sides. The long axis of the infarct is in the plane of the longest pleural surface with which it is in contact. The actual shadow may be round, wedge-shaped, or roughly triangular. It may assume the shape of the lingula or of the right middle lobe, or it may fill and obliterate a costophrenic sulcus if the lateral segment of a lower lobe is involved. Oblique views aided by fluoroscopic positioning may be necessary to bring out the relationship of the lesion to the pleura in many cases. The hilar aspect of the infarct is usually described as rounded or hump-shaped, rather than resembling the apex of a triangle. The appearance of a typical hump is unusual, if not rare, and almost any shape of opacity may be present. The amount of associated pleural effusion is usually not great, and multiple lesions may be seen in one or both lungs (Fig. 28-10). At first, the periphery of the lesion is rather hazy and poorly defined, but as time goes on it becomes more sharply outlined, and as it heals it gradually becomes smaller. The size of the infarct can vary greatly, from a small, faint opacity to the greater part of a lobe. The average size is about 3 to 5 cm. The pulmonary changes resolve rather slowly. The complete hemorrhagic necrotic infarct requires 4 weeks or more for complete resolution. When infarction is incomplete and there is no necrosis, the local findings are caused by edema and hemorrhage. This process may clear quickly (i.e., within 1 week) and leave no residue.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree