CHAPTER 48 Classical Concepts of Hydrocephalus
Hydrocephalus (“water on the brain”) is a condition in which there is excessive accumulation of cerebrospinal fluid (CSF) in the spaces of the intracranial compartment (cerebral ventricles, cisterns, and subarachnoid spaces). This accumulation of CSF results in distention of the cerebral ventricles and secondary increased intracranial pressure (ICP), which, if unresolved, leads to cerebral injury. Hydrocephalus results from an imbalance between the production of CSF, its circulation through the ventricles and subarachnoid spaces, and its reabsorption at distant sites, such as the arachnoid granulations of the dural venous sinuses.
According to the National Institute of Neurological Disorders and Stroke (NINDS), there is no current national registry or database for adult-onset hydrocephalus, resulting in a paucity of incidence and prevalence data in the medical literature.1
ADULT-ONSET OBSTRUCTIVE HYDROCEPHALUS
Unilateral distention of a single lateral ventricle may be seen with benign stenosis of the foramen of Monro, a lateral ventricular tumor, ventricular hemorrhage, or inflammatory ependymitis, leading to a chronically dilated ventricular body and contralateral shift of the septum pellucidum. A diffuse ventriculitis or ependymitis can produce segmental inflammatory coarctations of the ventricular spaces, with variable patterns of obstruction. One lateral ventricle may become selectively obstructed by masses that arise from the septum pellucidum or the anterior third ventricle, and obstruct the ipsilateral foramen of Monro. Lateral ventricular tumors in the adult may include ventricular meningioma, ependymoma, subependymoma, central neurocytoma, or choroid plexus metastases. Subependymal giant cell astrocytomas can be seen frequently in tuberous sclerosis patients in their adolescent to young adult years (Fig. 48-1).
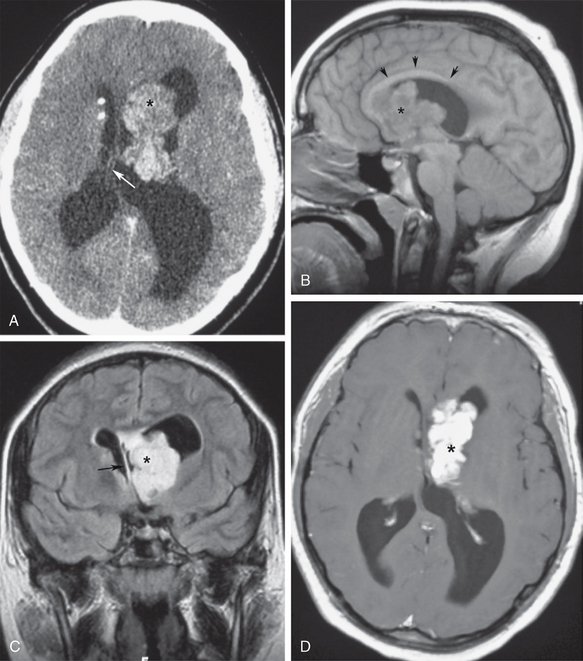
FIGURE 48-1 Asymmetric ventricular obstruction by subependymal giant cell astrocytoma in a young man with tuberous sclerosis. A, Contrast-enhanced axial CT (CECT) shows a lobulated left intraventricular mass (asterisk) with asymmetric obstruction of the left lateral ventricle, and shift of the septum pellucidum (arrow). Note the calcified subependymal nodules typical for tuberous sclerosis. B, Midsagittal T1W MR image shows the obstructing tumor (asterisk) immediately above the foramen of Monro. The corpus callosum is elevated by the obstructed lateral ventricle (arrows). C, Coronal T2W FLAIR section best demonstrates the asymmetric left tumor (asterisk), ventricular obstruction and displacement of the septum pellucidum to the right (arrow). Note the lack of periventricular interstitial edema, suggesting slow onset of ventricular distention. D, Gadolinium-enhanced axial T1W MR image shows an intense enhancement of the lobulated tumor (asterisk), with well-circumscribed margins.
Epidemiology
Intraventricular meningioma represents only 0.7% of all meningiomas, but it is the most common intraventricular tumor in the adult, typically occurring in patients older than 30 years. It has a peak age between 30 and 60 years and a mean age of 42 years. It affects females more than males in a 2:1 ratio. Its clinical presentation is usually headaches, nausea, and vomiting associated with increased ICP from obstructive hydrocephalus. Intraventricular meningiomas are believed to arise from arachnoidal cap cells trapped in the choroid plexus, tela choroidea, and velum interpositum, which explains its more frequent occurrence in the trigone of the lateral ventricles. Imaging features include a well-defined contour, hyperdensity compared with brain on simple CT, and intense homogeneous enhancement after contrast agent administration (Fig. 48-2). Intraparenchymal calcifications are visible in 50% of intraventricular meningiomas. MRI features are similar to those of convexity meningiomas, including a signal intensity that is isointense to hypointense to gray matter on T1-weighted images (T1W), and isointense to gray matter on T2-weighted (T2W) images, with intense enhancement after gadolinium administration. MR spectroscopy (MRS) reveals a spectral pattern similar to meningiomas at other sites, with decreased N-acetyl-aspartate and creatine levels and increased choline, lactate, lipid, and alanine levels.2
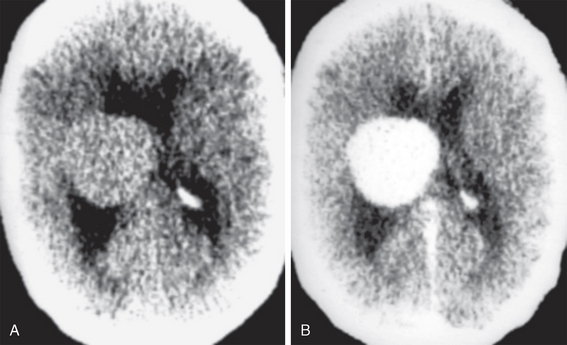
FIGURE 48-2 Intraventricular meningioma. A, Non-enhanced head CT (NCCT) shows a sharply marginated intraventricular mass, with homogeneous parenchyma that is isodense to cortex. B, Contrast-enhanced CT (CECT) shows intense enhancement of the entire lesion, without apparent ependymal wall invasion.
(Case courtesy of Dr. Eugene F. Binet. Augusta, GA.)
Although frequently a pediatric brain tumor, ependymoma can occur at any age, with a documented age range of 1 month to 81 years. Although most posterior fossa ependymomas occur in the pediatric age group, supratentorial ependymomas have a higher mean age at presentation (18 to 24 years), with 42% of ependymomas occurring in the third and lateral ventricles. Although tumor recurrence is common in all types of ependymomas, adult patients with supratentorial ependymomas have a better survival rate than those with posterior fossa ependymomas, with 5- and 10-year survival rates in adults of 57.1% and 45%, respectively.3 Intraventricular ependymomas are hypodense to isodense on noncontrast CT (NCCT), with punctate calcifications in 40% to 80% of cases. Contrast enhancement is variable, outlining frequent intratumoral cysts. On MRI intraventricular ependymomas are T1 isointense and T2 hyperintense to cerebral cortex, with a heterogeneous appearance proportional to their calcification, hemorrhage, and cystic components. Their gadolinium enhancement is variable, depending upon the extent of their solid tumor components.
Subependymomas and central neurocytomas are less common ventricular tumors, both having a predilection for the anterior lateral ventricles in the vicinity of the foramen of Monro. Subependymoma is also found in the fourth ventricle. Both lesions may be heterogeneous with predominant cystic components and variable patterns of enhancement. When clinically apparent, both lesions typically present with obstructive hydrocephalus. Subependymomas are more common in older adults, whereas neurocytomas are more common in patients younger than age 40 years, with an age range of 17 to 53 years (Fig. 48-3).4,5
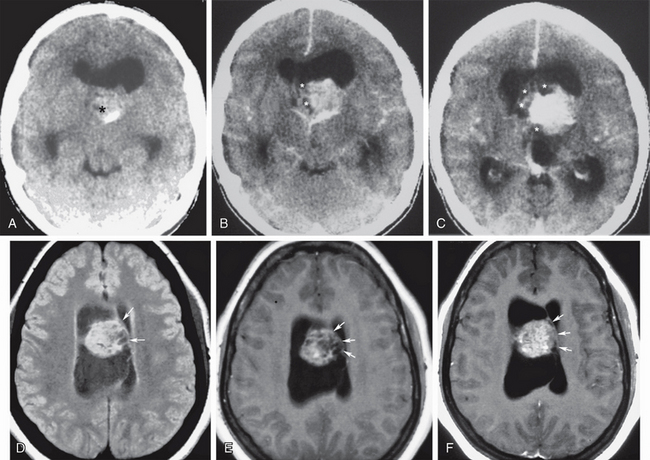
FIGURE 48-3 Central neurocytoma. A 30-year-old woman presented with chronic headaches of progressive severity and papilledema. A, Nonenhanced head CT (NCCT) shows a midline circumscribed isodense to slightly hyperdense intraventricular mass (asterisk) with a peripheral calcification, obstructing both lateral ventricles at the foramina of Monro. B and C, Contrast-enhanced sequential axial CT images (CECT) show intense parenchymal enhancement mixed with more peripheral areas of cystic change (asterisks). D to F, Different case of central neurocytoma FLAIR T2W (D), T1W (E), and gadolinium-enhanced T1W (F) axial MR images at the same level showing the typical location of this intraventricular tumor, projecting from the septum pellucidum (arrows). Note the microcystic tumor appearance shown as areas of lack of contrast enhancement on F.
Ventricular obstruction at the foramen of Monro can be triggered from the third ventricle lumen, typically producing symmetric obstruction of both lateral ventricles. Rapid obstruction at the level of the third ventricle in the adult may occur from colloid cysts of the third ventricle, intraventricular neurocysticercosis cysts, or sellar/suprasellar lesions that bulge upward to compress the ventricle (craniopharyngiomas, pituitary apoplexy with acute hemorrhagic enlargement of the gland) (Fig. 48-4). Sudden obstruction of the lateral ventricles from positional change of a third ventricular colloid cyst may be a catastrophic event, with plugging of the foramen of Monro leading to rapid development of intracranial hypertension, which if not promptly recognized and treated may result in sudden death (Fig. 48-5).6 Third ventricular obstruction of more chronic insidious nature may result from slow-growing tumors, such as ependymomas, hypothalamic gliomas or pineal lesions, craniopharyngiomas, slowly enlarging pituitary adenomas, or giant basilar tip aneurysms.
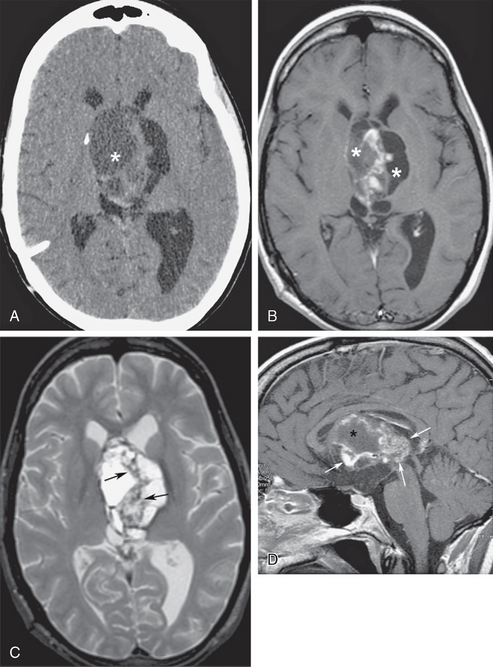
FIGURE 48-4 Obstructive hydrocephalus from suprasellar extension of craniopharyngioma. A, Nonenhanced axial CT (NCCT) at the level of the foramen of Monro shows a peripherally calcified cystic mass (asterisk) that obstructs the lateral ventricles. B, Axial T1W MR image at the same level as A shows varying hyperintensity of the signal from the cystic components (asterisks) due to increased protein content. C, Heterogeneous signal of the septations on fast spin-echo T2W axial MR image may be due to fibrotic changes, calcifications, and/or inspissated proteinaceous debris (arrows). D, Gadolinium-enhanced midsagittal T1W MR image shows areas of heterogeneous enhancement within the solid components of the craniopharyngioma (arrows), outlining cystic spaces (asterisk).
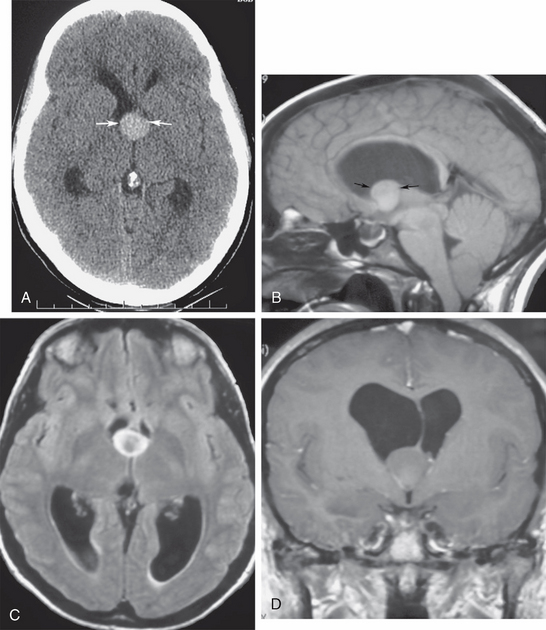
FIGURE 48-5 Colloid cyst of third ventricle, with severe obstructive hydrocephalus. A 22-year-old woman presented with acute onset of severe headache. A, Nonenhanced axial CT (NCCT) shows a midline oval hyperdense mass at the foramen of Monro (arrows), with obstructed lateral ventricles above the lesion. B, Midsagittal T1W MR image depicts the classic location of the colloid cyst at the anterior roof of the third ventricle (arrows). Note the T1 shortening of its protein content, with signal intensity resulting from variable protein concentrations. C, Axial T2W FLAIR section shows that the cyst is hyperintense to normal ventricular CSF. D, Coronal gadolinium-enhanced T1W MR image depicts the deformity of the cyst where it wedges against the undersurface of the septum pellucidum and the foramen of Monro, entrapping the lateral ventricles.
Adult-onset obstruction of the aqueduct of Sylvius may be due to congenital aqueductal insufficiency with late decompensation, inflammatory ependymitis with obstruction, intraventricular hemorrhage, or extrinsic compression from tumors, abscesses, or tumefactive perivascular spaces. Any of these may present with insidious headaches or sudden onset of intracranial hypertension (Fig. 48-6). Tectal gliomas, pineal tumors and third ventricular tumors, although more frequent in the pediatric age group, also cause adult-onset aqueductal insufficiency (see Fig. 48-6).
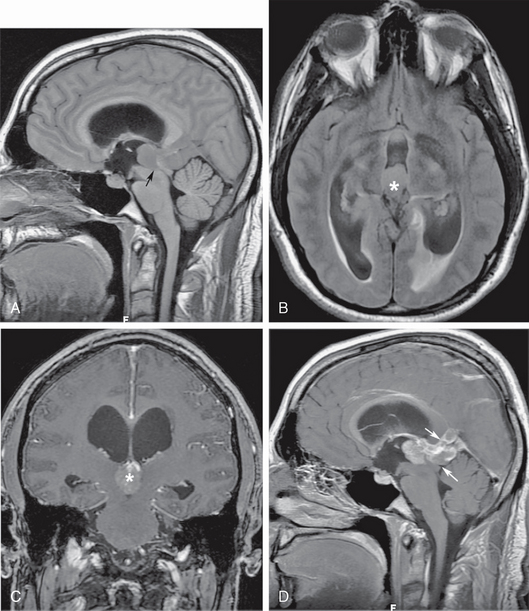
FIGURE 48-6 Pinea germinoma with aqueductal obstruction. A, Isointense pineal recess mass blocks the entrance to the cerebral aqueduct (arrow), resulting in supra-aqueductal ventricular distention. B, Axial T2 FLAIR section shows the distended third ventricle, with subependymal interstitial edema around the ventricular atria (asterisk). C, Coronal gadolinium-enhanced T1W MR image shows intense tumor enhancement (asterisk) filling the lumen of the posterior third ventricle. D, Sagittal gadolinium-enhanced T1W image better depicts the extension of germinoma into the quadrigeminal plate cistern and the region of the vein of Galen (arrows).
In the adult, fourth ventricular obstruction can be seen in cases of inflammatory ependymitis or basal meningitis obstructing the outlet foramina of the fourth ventricle (Fig. 48-7). In some cases, inflammatory or post-hemorrhagic ependymitis may “trap” the fourth ventricle by obstructing the aqueduct and the outlet foramina. This “trapped fourth ventricle” is recognized by its lack of decompression after lateral ventricular shunt placement. It is treated by placing a separate catheter into the fourth ventricle. Obstructive hydrocephalus can also occur from compression of the fourth ventricle by masses from the cerebellar hemispheres, such as hemangioblastomas or astrocytomas, cerebellar metastases, hematomas, or acute infarcts (Fig. 48-8). Large extra-axial posterior fossa masses may also compress and deform the cerebellum and brain stem, impairing CSF outflow. This secondary effect is seen most frequently with large cerebellopontine angle meningiomas or vestibular schwannomas, posterior fossa subdural or epidural hematomas, or large exophytic skull base chordomas or chondrosarcomas (Fig. 48-9).
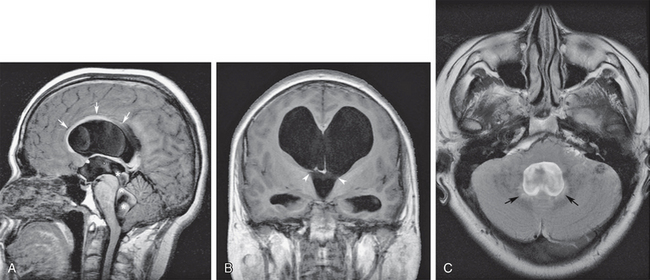
FIGURE 48-7 Obstructive hydrocephalus at the level of the fourth ventricular outlet foramina from prior meningitis. A, Midsagittal T1W MR image shows severe obstructive dilatation of the entire ventricular system. There is arcuate elevation of the corpus callosum from lateral ventricular distention (arrows) and flow-related CSF dephasing (asterisks) across the dilated cerebral aqueduct from hyperdynamic pulsatile flow. B, Coronal T1W MR image shows the distended lateral and third ventricles with bilateral expansion of the foramen of Monro (between arrowheads). C, Axial T2 FLAIR image at the level of the distended fourth ventricle shows subependymal interstitial edema (arrows) and effacement of the subarachnoid spaces around the cerebellum. Compare with the downward herniation of the cerebellar tonsils seen in A.
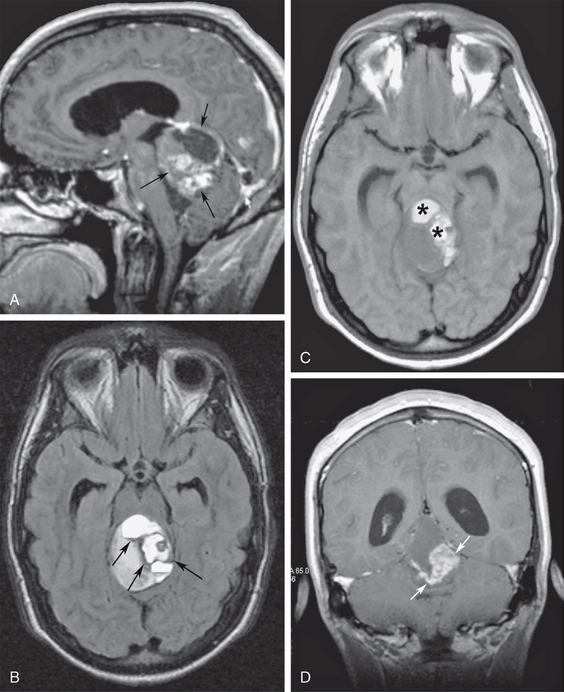
FIGURE 48-8 Superior vermian cerebellar astrocytoma with tectal compression. A, Midsagittal gadolinium-enhanced T1W MR image shows a large, heterogeneous, enhancing vermian mass (arrows) compressing the tectum of the midbrain and the upper wall of the fourth ventricle. B, Axial T2 FLAIR MR image at the midbrain level shows hemosiderin along the walls of the tumor cyst (arrows). Note the distended temporal horns and infundibular recess of the third ventricle. C, Axial T1W image at the same level as B shows hyperintense signal of the tumor cysts (asterisks) in keeping with hemorrhagic behavior (free methemoglobin). D, Coronal contrast-enhanced T1W MR image identifies the solid tumor components (arrows).
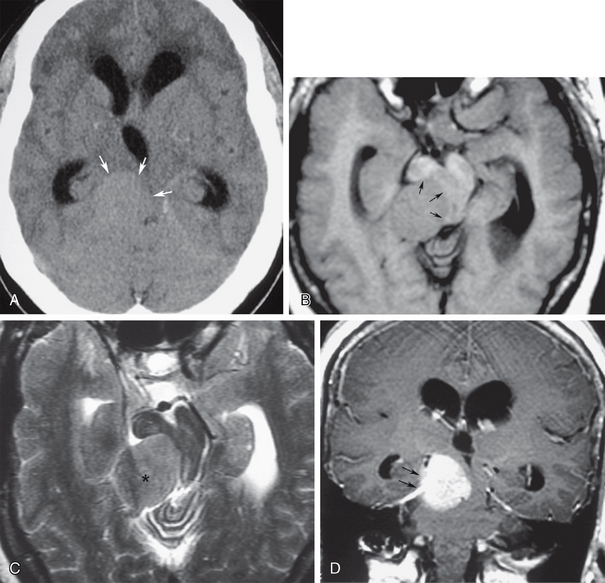
FIGURE 48-9 Right tentorial meningioma producing obstructive hydrocephalus. A, Nonenhanced axial CT (NCCT) shows a right tentorial isodense extra-axial mass (arrows) compressing the midbrain toward the left, with supra-aqueductal ventricular obstruction. B, Axial T1W MR image at the same level as A shows an isointense mass projecting from the right tentorium severely compressing and deforming the midbrain and aqueduct (arrows). C, Axial T2W MR image at the same level shows that the mass is isointense with cerebral cortex, consistent with meningioma (asterisk). D, Coronal gadolinium-enhanced T1W MR image shows best the right tentorial origin (arrows) of the intensely enhancing meningioma.
Segmental obstruction of portions of the ventricular system may also occur at any level due to inflammatory ependymitis, leading to ventricular scars and entrapment (Fig. 48-10). Subependymal tumor infiltration from gliomas, primary central nervous system lymphoma, primary or metastatic choroid plexus lesions, or atypical teratoid rhabdoid tumors may also cause ventricular entrapment, but much less frequently than inflammatory disease (Fig. 48-11).
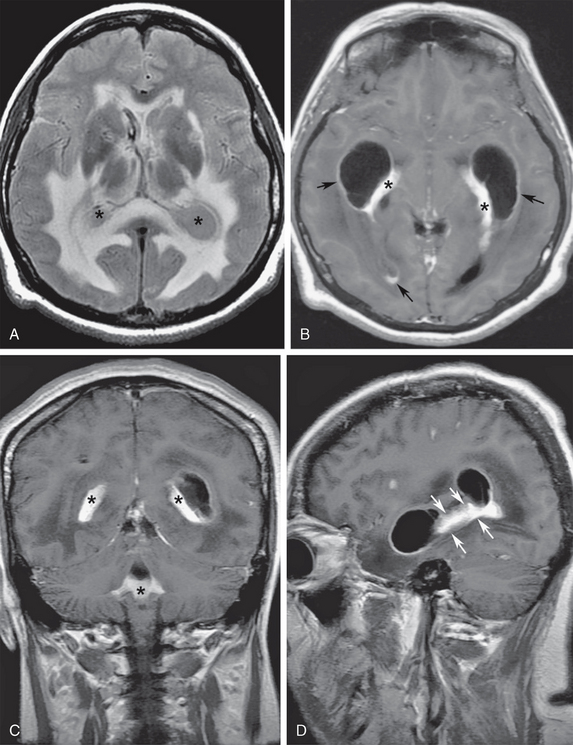
FIGURE 48-10 A 35-year-old woman presented with severe headaches for 4 months and signs of meningeal irritation and papilledema over the last week, progressing to stupor. The final diagnosis was cryptococcal choroid plexitis and ependymitis. A, Axial T2 FLAIR image shows entrapment of the temporal horns, collapsed third ventricle, and increased signal of the ventricular CSF (asterisks). Note the prominent subependymal interstitial white matter edema. B, Axial gadolinium-enhanced T1W MR image shows abnormal nodular thickening of the choroid plexus (asterisks) and diffuse ependymal enhancement (arrows) in the temporal and occipital horns of the lateral ventricles and in the third ventricle. C, Coronal gadolinium-enhanced image shows extension of the choroid plexitis (asterisks) within the fourth ventricle. D, Sagittal gadolinium-enhanced T1W MR image of the left temporal horn shows ependymal retractions (arrows) with segmental ventricular entrapment.
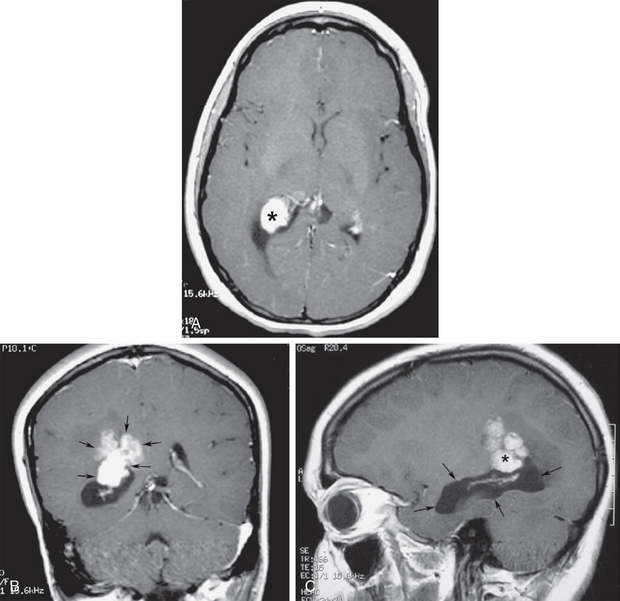
FIGURE 48-11 Choroid plexus carcinoma with temporal horn entrapment. A, Gadolinium-enhanced axial T1W MR image shows an enhancing mass (asterisk) expanding the choroid glomus at the trigone. B, Gadolinium-enhanced coronal T1W image shows the mass invading through the ependymal margin into the parietal subependymal white matter (arrows). C, Gadolinium-enhanced sagittal T1W MR image shows entrapment of the right temporal horn (arrows) by the trigonal mass (asterisk).
Clinical Presentation
Patients with acute onset of ventricular obstruction and sudden hydrocephalus present with rapid onset of severe headaches, progressing to obtundation and death if prompt diagnosis and decompression are not achieved. The classic example is the colloid cyst of the third ventricle positionally herniating into the foramen of Monro and acutely obstructing the lateral ventricles (see Fig. 48-5). In patients presenting acutely, CT is often the only examination obtained prior to emergency surgical decompression of the obstructed ventricles.
Patients with slower evolution of ventricular obstruction may present in a more subtle fashion, with headaches of longer evolution before the appearance of clinically observable neurologic findings. Problems with visual acuity or extraocular muscles (sixth cranial nerve palsy) and papilledema are typically already present on funduscopic examination. Head CT is the first line of diagnosis, showing the distribution of the ventricular obstruction and variable patterns of periventricular interstitial fluid accumulation. CT may or may not display the actual cause of the obstruction. However, MRI of the brain, typically with gadolinium enhancement, is the examination of choice to better evaluate the etiology of the chronic ventricular distention.
Pathophysiology
Approximately 85% of the CSF is produced by active transport in the choroid plexus, the highly vascular neuroepithelial tissue responsible for the production of CSF within the ventricular system. The choroid plexus is found within the lateral ventricles, the roof of the third ventricle, and the medullary velum of the fourth ventricle, frequently extending out through the fourth ventricle outlet foramina into the basal cisterns. The remaining CSF appears to be formed by active transport across capillary endothelium into the interstitial spaces of the white matter. This accounts for most of the interstitial fluid seen by MRI along the subependymal periventricular spaces. The production of CSF is about 600 mL every 24 hours, with this production being relatively stable from childhood to old age. The combined total CSF capacity of the ventricular and craniospinal subarachnoid spaces in the adult is approximately 150 mL, implying a complete replenishment of the entire CSF volume by freshly produced CSF about 3-4 times every 24 hours. Obstruction of the ventricular CSF flow thus results in rapid ventricular dilatation, leading to intracranial hypertension. CSF production remains constant in spite of increased ICP, stopping only after complete arrest of intracranial blood flow with depletion of the cellular level energy metabolism necessary to produce CSF by active transport.7
Imaging
CT
CT remains the first line of diagnosis for the evaluation of acute intracranial hypertension and is primarily used for hyperacute surgical management decisions. Modern multidetector CT also allows the display of highly detailed brain/ventricular anatomy in multiplanar (coronal sagittal or oblique) projections, allowing a more comprehensive evaluation of the acute surgical lesion in a fashion only possible previously by MRI. The ability to perform CT angiography and CT perfusion at the initial evaluation provides the radiologist and clinicians valuable functional information that frequently alters the management of the lesion causing the obstructive hydrocephalus (subarachnoid hemorrhage from a ruptured aneurysm, tumor, or meningitis with acute hydrocephalus) (Fig. 48-12).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



