Riccardo Lencioni • Marcelo Guimaraes
Hepatocellular carcinoma (HCC) is the second leading cause of cancer-related death worldwide.1 Unlike most solid cancers, future incidence and mortality rates for HCC were projected to largely increase in several regions around the world over the next 20 years.2,3 A careful multidisciplinary assessment of tumor characteristics, liver function, and physical status is required for proper therapeutic management of patients with HCC.4–7 Candidates for resection must be carefully selected to minimize the risk of postoperative liver failure and improve long-term results. Access to liver transplantation has to be balanced between precise estimation of survival contouring individual tumor characteristics and organ availability. When surgical options are precluded, interventional locoregional treatments are recommended as the most appropriate therapeutic choice for patients whose disease is limited to the liver.4–7
Image-guided tumor ablation is the first-line treatment for nonsurgical patients with early-stage HCC and is considered a potentially radical therapy in properly selected candidates.8–10 Several reports have shown that the long-term survival of patients with compensated cirrhosis and small solitary tumors who received radiofrequency ablation (RFA) as the sole first-line anticancer therapy is similar to that reported in surgical series.11,12 For patients presenting with more advanced or multinodular HCC—a common scenario due to the propensity of HCC to invade portal vein branches and to produce intrahepatic metastases—and relatively preserved liver function, transcatheter arterial chemoembolization (TACE) is the current standard of care. TACE is actually the most popular treatment for HCC worldwide.13 Radioembolization with yttrium 90 microspheres is attracting increasing attention as a potential alternate treatment that seems to have potential to provide clinical benefit even in the complex setting of patients presenting with macroscopic vascular invasion.14
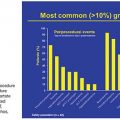
Despite their leading role in the clinical management of HCC, locoregional interventional therapies have limitations. The ability of image-guided ablation techniques to achieve complete tumor eradication appears to strongly depend on tumor size and location.15 Even in those patients in whom successful tumor ablation has been achieved, the rate of tumor recurrence due to the emergence of new HCC lesions exceeds 80% at 5 years, similar to postresection figures.16 On the other hand, in patients with large or multinodular tumor at the intermediate-stage HCC who received TACE, tumor recurrence or progression is almost inevitable. In randomized controlled trials, a sustained response lasting more than 3 to 6 months was observed in only 28% to 35% of the patients who received conventional TACE; in nonresponders, no survival benefit was identified compared to best supportive care.17,18 Even in those patients in whom initial response was achieved, the 3-year cumulative rate of intrahepatic recurrence reached 65%, with recurrent tumor showing significantly shorter median doubling time.19
Several combined treatment strategies have been used in an attempt to overcome some of these limitations. These include various combinations of locoregional interventions as well as the association of locoregional and systemic therapies. This chapter is focused on discussing the potential synergies of TACE with either local ablation or molecular targeted agents, which have been the most extensively described combination approaches. Despite combined treatments are widely used in clinical practice, unequivocal evidence of clinical benefit associated with such strategies is still lacking as no robust phase III randomized clinical trials have been completed so far.
COMBINED TRANSCATHETER ARTERIAL CHEMOEMBOLIZATION AND LOCAL ABLATIVE THERAPY
RFA is currently established as the standard ablative modality for early-stage HCC.20,21 Randomized trials comparing RFA versus the seminal percutaneous technique—ethanol injection—showed that RFA has higher local anticancer effect, leading to a better control of the disease and improved survival.22–26 Nevertheless, histologic studies performed in liver specimens of patients who underwent RFA as bridge treatment for transplantation showed that the rate of complete tumor eradication highly depends on tumor size and presence of large (≥3 mm) abutting vessels. In fact, vessels adjacent to the target tumor cause heat loss due to perfusion-mediated tissue cooling within the area to be ablated. In one study, the rate of complete tumor necrosis was 50% or less in tumor exceeding 3 cm in diameter or in perivascular location.15
Several attempts have been made to increase the effect of RFA in HCC treatment. Because heat efficiency is the difference between the amount of heat produced and the amount of heat lost, most investigators devoted their attention to strategies that aim primarily at minimizing heat loss due to perfusion-mediated tissue cooling.27 When a laparotomy or a laparoscopy approach is used to perform RFA, heat loss can be minimized by performing a Pringle maneuver.27 In percutaneous procedures, given that HCC is mostly nourished by the hepatic artery, a combination of RFA and balloon catheter occlusion of the tumor arterial supply or prior transcatheter arterial embolization or chemoembolization has been used to increase heat efficiency.28–31 The combination of TACE and RFA did not show significant clinical benefit when applied to the treatment of small (≤3 cm) HCC. In a randomized study, the rates of local tumor progression and overall survival did not differ between the combined treatment group as compared to the RFA-alone treatment group.32 However, such an approach did show significant advantages both in terms of local tumor control and survival when used in intermediate-sized (3 to 5 cm) HCC33,34 as well as in recurrent HCC after prior hepatectomy.35
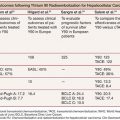
The largest randomized clinical trials conducted so far included 189 patients with HCC less than 7 cm. Patients were randomly assigned to receive TACE combined with RFA (TACE–RFA; n = 94) or RFA alone (n = 95). The primary end point was overall survival. The secondary end point was recurrence-free survival, and the tertiary end point was adverse effects. The 1-, 3-, and 4-year overall survivals for the TACE–RFA group and the RFA group were 92.6%, 66.6%, and 61.8% and 85.3%, 59%, and 45.0%, respectively. The corresponding recurrence-free survivals were 79.4%, 60.6%, and 54.8% and 66.7%, 44.2%, and 38.9%, respectively. Patients in the TACE–RFA group had better overall survival and recurrence-free survival than patients in the RFA group (hazard ratio, 0.525; 95% CI, 0.335 to 0.822; P = .002; hazard ratio, 0.575; 95% CI, 0.374 to 0.897; P = .009, respectively). On logistic regression analyses, treatment allocation, tumor size, and tumor number were significant prognostic factors for overall survival, whereas treatment allocation and tumor number were significant prognostic factors for recurrence-free survival.36
The strategy of performing TACE first followed by RFA has the advantages of cutting the arterial blood supply, which reduces the “heat sink effect” during the RFA. Also, it is expected to have an increase in the size of the RFA ablation zone. This can be beneficial to guarantee a good surgical margin post ablation, but it can also be dangerous in case the ablation is performed close to a liver structure (confluence of the bile ducts) and to an adjacent organ (gallbladder, diaphragm, stomach, colon) in which undesired heat-induced injury may occur. TACE first also allows the delivery of high concentration of the chemotherapeutic agent to the entire tumor. In addition, it has been reported that the heat generated by the RFA post TACE could sensitize the chemotherapeutic agent.37
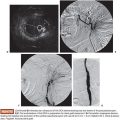
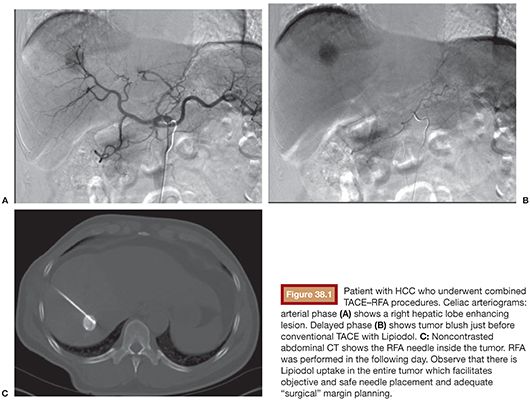
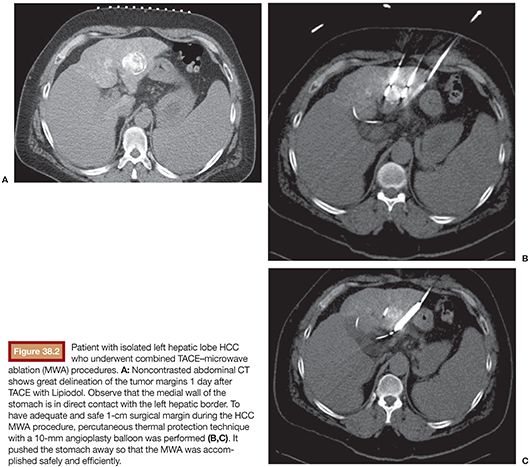
Currently, there is no evidence determining if conventional or drug-eluting bead (DEB) TACE is better when used in combination with RFA. However, if the RFA is performed under computed tomography (CT) guidance, the use of TACE with Lipiodol is helpful in identifying the nodule location (Fig. 38.1A–C). It also delineates the tumor border precisely (which is key to plan a 1-cm surgical margin around the tumor) and increases accuracy for RFA needle deployment and for multiple overlapping ablations planning. This feature is especially important for tumors larger than 2 or 3 cm and for those in risky locations. In this case, percutaneous thermal protective techniques may be used (Figs. 38.2A–C and 38.3A–F).

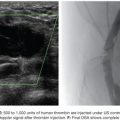
Another debatable question is the time interval between TACE and RFA. In theory, the time between the two procedures should be minimized to prevent hypoxia-induced neoangiogenesis. Typically, the tumor recruitment of existing and/or the formation of new arterial blood supply takes place between 2 and 3 weeks. One of this chapter authors (M.G.) routinely performs conventional TACE procedure followed by CT-guided RFA in the next day (“back-to-back technique”). This strategy has been well tolerated by Child-Pugh A and B patients. Also, it has optimized resources and minimized patient’s dislocations and hospital admission time. These data, not published yet, have demonstrated encouraging results including large HCCs (Figs. 38.4A–F and 38.5A–G) with less than 1% tumor recurrence rate, increased patient’s survival, and without major complications. Although TACE followed by RFA has been the most popular combination, alternate strategies have been investigated. In particular, DEB-TACE has been performed after RFA, rather than before, following a different rationale.38 In a standard RFA, one can take advantage of only those temperatures that are sufficient by themselves to induce coagulative necrosis (>50° to 55°C). However, there are large zones of sublethal heating created during radiofrequency (RF) application in tissues surrounding the electrode that are not being used to achieve sustained treatment effect. Experimental studies in animal tumor models have shown that lowering the temperature threshold at which cell death occurs by combining sublethal temperature with cell exposure to chemotherapeutic agents increases tumor necrosis, apparently occurring in tissues heated to 45° to 50°C.39,40 It has been hypothesized that the administration of DEB to tumors incompletely killed by RFA could increase the anticancer effect as a result of both the delivery of highly concentrated doxorubicin into a relatively small volume of residual viable neoplastic tissue and the reduced cell resistance to the drug caused by the exposure to sublethal heating.38

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree