Jean Noel Buy1 and Michel Ghossain2
(1)
Service Radiologie, Hopital Hotel-Dieu, Paris, France
(2)
Department of Radiology, Hotel Dieu de France, Beirut, Lebanon
32.3 Rupture or Leakage
32.3.1 The Cyst
32.3.2 The Hemoperitoneum
32.3.3 The Peritoneum
32.4 Adnexal Torsion
32.4.2 Early Phase (Edema)
32.4.3 Hemorrhagic Necrosis
32.4.4 Associated Findings
32.4.5 Global Approach
32.4.6 Spontaneous Detorsion
32.4.7 Massive Ovarian Edema
32.4.8 Diffusion Sequences
32.5 Necrosis
32.6 Infection
32.7 Fistula
32.8 Hernia
Abstract
Ovarian masses are usually asymptomatic and may grow to significant size before they can be palpated and give rise to symptoms. Intracystic hemorrhage, rupture or leakage, and torsion are the most frequent complications; infection, necrosis, fistula, and hernia are rare.
Ovarian masses are usually asymptomatic and may grow to significant size before they can be palpated and give rise to symptoms. Intracystic hemorrhage, rupture or leakage, and torsion are the most frequent complications; infection, necrosis, fistula, and hernia are rare.
This chapter will make a rapid recall on hemorrhage in cystic portion, hemorrhage in solid portion, and paraneoplastic syndromes, which are treated extensively in other chapters; it will focus on more severe complications, i.e., leakage (or rupture), torsion, necrosis, infection, fistula, and hernia.
32.1 Intracystic Hemorrhage
Intracystic hemorrhage usually occurs in functional cyst or corpus luteum cyst, and for a lot of authors, “hemorrhagic cyst” is the synonym of “functional hemorrhagic cyst” or “corpus luteum hemorrhagic cyst.” However, one must keep in mind that any tumor either benign or malignant can show intracystic hemorrhage (Table 32.1) (Fig. 32.1).
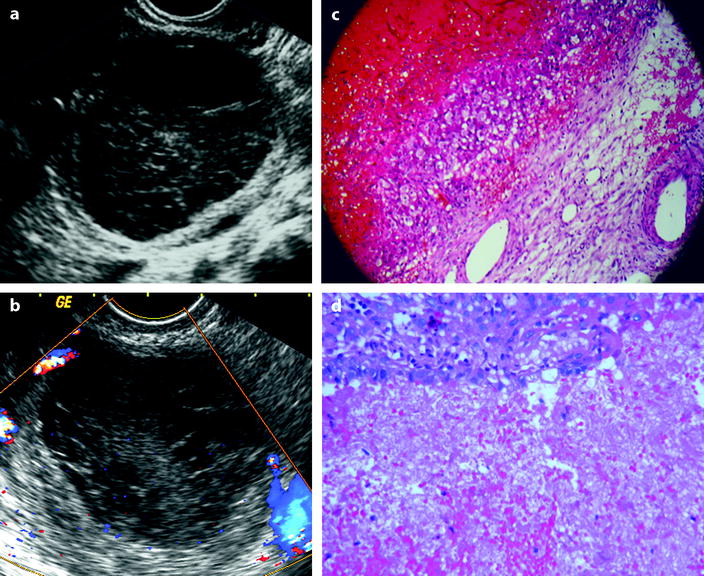
Table 32.1
Ultrasound pattern of FHC and CLHC
Loculation |
– Usually unique and unilocular |
– Rarely multilocular, multiple, and/or bilateral (after ovarian stimulation, in newborn, perimenarchal, and perimenopausal age) |
Intracystic pattern |
Suggestive |
– Interdigitating fibrin septations |
Thin (fishnet appearance) |
Coarse (like a sponge) |
Spaced (simulating septa or floating membranes) |
– Clot |
Central or eccentric |
Stellate, with digitations, or well circumscribed |
– Fluid level |
– Small echo-free area(s) on diffuse echoic background |
Uncommon |
Diffuse hypoechoic |
Diffuse hyperechoic |
Complex pattern |
Any combination of the above patterns |
Wall |
– Thick, hyperechoic, and vascular in CLHC |
– Thinner, less hyperechoic, and less vascular in FHC |
Doppler |
– Avascular intracystic content |
– Vascularized wall |
– Resistivity index may be low (<0.4) in CLHC |
Differential diagnosis |
– Endometrioma (especially if fluid level or homogeneous isoechoic pattern) |
– Dermoid cyst (especially if fluid level or homogeneous hyperechoic pattern) |
– Malignant tumor with solid vascularized component if no Doppler study or if Doppler study technically limited (transabdominal approach or bad technical conditions) |
– Any cystic tumor with intracystic hemorrhage |
– Any solid necrotic tumor |
– Abscess |
– Ectopic pregnancy |

Fig. 32.1
FHC versus hemorrhagic cystadenoma: (a) and (c) correspond to a FHC that has been proved surgically. At histology, the wall of the cyst is lined by granular cells. (b) and (d) correspond to a hemorrhagic cystadenoma without vegetation or solid tissue. At histology, the epithelium of the cyst is abraded but still recognizable. At ultrasound, both cysts contain echoic material avascular on Doppler study, and no criteria can distinguish them
In malignant tumors, hemorrhage in cystic and/or solid portion is frequent, but the features of malignancy usually predominate. Hemorrhage in solid portion of benign tumors is rare.
The patterns of functional hemorrhagic cyst (FHC) and corpus luteum hemorrhagic cyst (CLHC) have been extensively treated in Chap. 7. Intracystic hemorrhage is often asymptomatic, and this chapter will focus on more severe complications.
32.2 Hemorrhage in a Solid Portion of a Tumor
It is a common finding in primary and secondary malignant tumors [5, 6]. In the absence of torsion, this finding is rare in benign tumors [7].
This finding can be considered as part of tumor description and will not be treated in this chapter but in each tumor description.
32.3 Rupture or Leakage
Rupture of a cyst is usually spontaneous, sometimes secondary to trauma such as intercourse or blunt trauma like in a motor vehicle accident. Rarely, it could be secondary to infection.
Rupture is usually associated with FHC, but one must be aware that paraovarian cysts, endometrioid cysts, and ovarian tumors may rupture (Table 32.2) (Figs. 32.2 and 32.3).
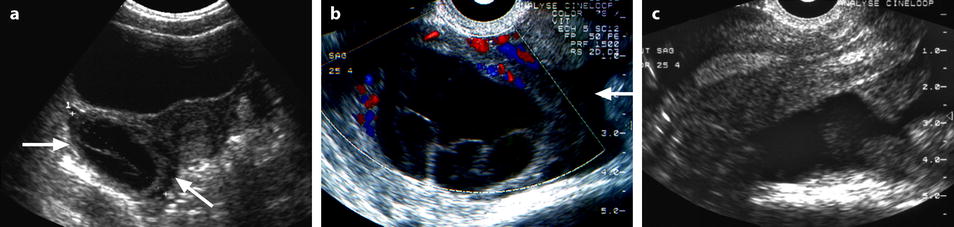
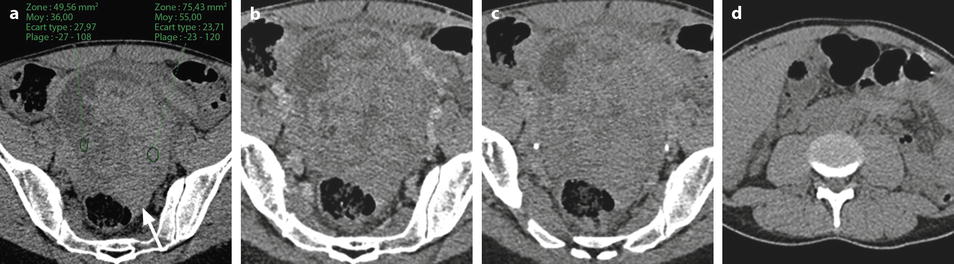
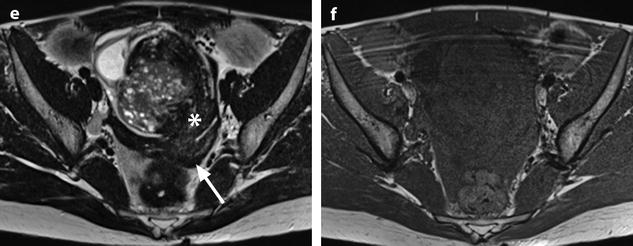
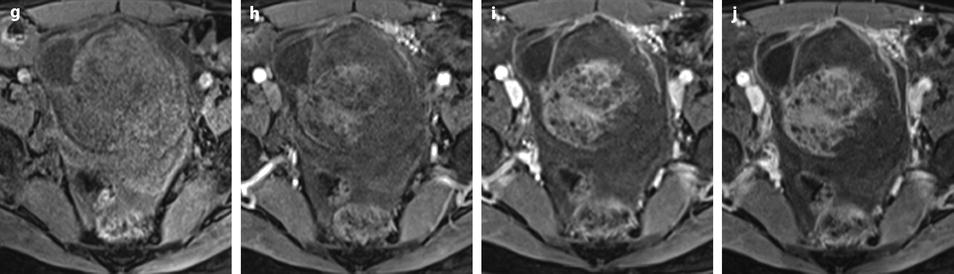
Table 32.2
Ultrasound patterns of ruptured cysts
The cyst |
– Partially or totally collapsed |
– Well circumscribed |
– Nonvisualized |
Intracystic pattern |
– Same as FHC or CLHC |
– May be anechoic |
– May depend of the nature of the cyst |
Hemoperitoneum |
– Anechoic |
– Hyperechoic |
– Mixed anechoic and hyperechoic |
Histology |
– Usually FHC or CLHC |
– Any benign or malignant cystic tumor |
Differential diagnosis |
– Differentiating FHC and CLHC from cystic benign or malignant tumor (follow-up or surgery) |
– Ectopic pregnancy |

Fig. 32.2
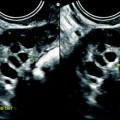
Ruptured FHC with spontaneous regression. (a) Transabdominal ultrasound in a young woman with pelvic pain discloses a right ovarian cyst (horizontal arrow) with echoic intracystic materials. A small peritoneal effusion is barely seen (oblique arrow). (b) Endovaginal ultrasound with Doppler shows no color flow in the intracystic interlaced fibrin septa. A small peritoneal effusion (arrow) is seen abutting the inner side of the cyst, but the site of rupture could not be clearly determined. (c) Sagittal view of the pouch of Douglas better displays the peritoneal effusion that contains echoic materials. Follow-up ultrasound showed complete disappearance of all findings confirming the diagnosis of FHC



Fig. 32.3
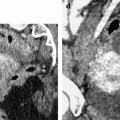
Ruptured granulosa tumor. A 33-year-old woman with sudden pain onset. CT scans before injection (a) at the portal phase (b) and at 8 min (c) show a pelvic mass with low-density liquid, solid portions that enhance after injection (small ROI in a: 36 UH before, 78 UH at portal phase, and 66 UH at 8 min), and high-density liquid that does not enhance (large ROI in a: 55 UH before injection). Only contrast uptake allows to differentiate vascularized tissue from hemorrhage. High-deionsity liquid is also present in the pouch of Douglas which is difficult to differentiate from the mass (arrow in a). Liquid is also present in the upper abdomen (d) Fig. 32.3 (continued) An MRI is performed 2 days later. (e) T2-weighted image shows the tumor to contain a solid portion with small cystic components; the ruptured wall is well disclosed between the intracystic hypointense hemorrhagic content (asterisk) and the hypointense hemorrhagic effusion (arrow). The intracystic and extracystic hemorrhage is hypointense on T1 (f) and T1 fat saturation images. Dynamic MRI images before injection (g) and at the arterial (h), portal (i), and late (j) phases well disclose the solid component and the ruptured wall
32.3.1 The Cyst
The cyst may conserve a round appearance, and the main finding of rupture is hemoperitoneum (Figs. 32.4 and 32.5). The absence of collapse of the cyst may be due to the presence of an intraluminal clot, the obstruction of the break by a clot, or rupture of a vessel on the external wall of the cyst.
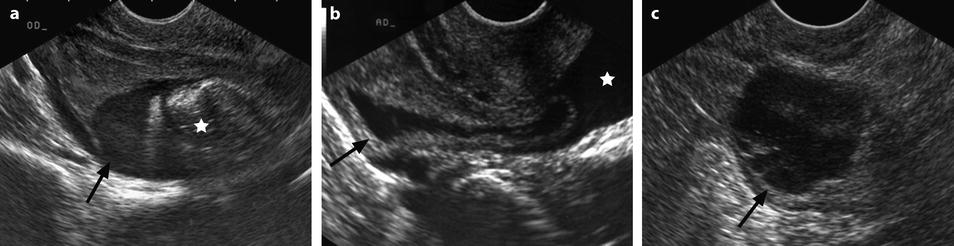
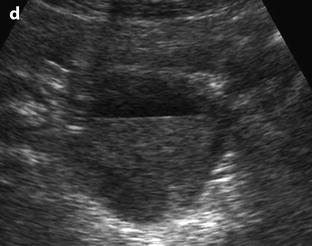
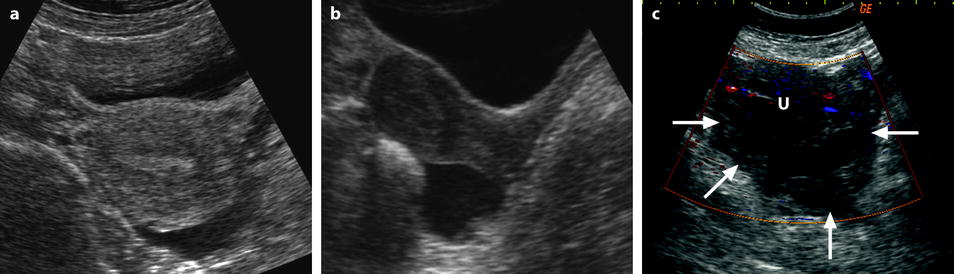
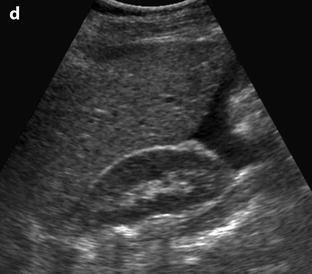


Fig. 32.4
Different patterns of a ruptured cyst on endovaginal (a, b, and c) and transabdominal (d) ultrasound. (a) Obvious rupture of a FHC with bowel (asterisk) coming in and out the lumen in real-time ultrasound; cyst wall (arrow). (b) Collapsed FHC cyst (arrow) adjacent to peritoneal effusion (asterisk) but the site of the rupture cannot be identified. (c) Still round but slightly collapsed cyst (arrow) containing avascular echoic material due to hemorrhage. The site of the rupture could not be identified. At histology a ruptured serous cystadenoma was disclosed. (d) Still round ruptured FHC cyst with liquid-debris level. The site of the rupture could not be identified (same patient as in Fig. 32.6)


Fig. 32.5
Different patterns of hemoperitoneum. Hemoperitoneum, either secondary to ruptured cyst or ectopic pregnancy, may be anechoic, echoic, or of mixed echostructure. On transabdominal approach, the fluid is mainly accumulated in the pouch of Douglas. When anechoic or slightly echoic, it is easily detected either on transverse (a) or sagittal (b) view. (c) When echoic, it is more subtle to visualize and is best disclosed on transverse views as a more or less heterogeneous echoic structure (arrows), avascular on Doppler, surrounding the uterus (letter u) on its posterior and both left and right sides. Ovaries are often difficult to visualize in this magma often called hematoma. (d) One must always look for fluid in the upper abdomen especially in the Morrison pouch. Fluid in the upper abdomen is indicative of an important hemoperitoneum
The cyst may appear collapsed with or without visualization of the rupture. Sometimes bowel loops may be seen inside the lumen. The intracystic pattern is usually that of FHC or CLHC, may be anechoic on ultrasound, or depends of the nature of the cyst (Table 32.2).
In some cases, the ruptured cyst is not visualized at all either because it is entirely collapsed or because adjacent clots in the associated hemoperitoneum prevent visualizing the ovary and/or the cyst.
32.3.2 The Hemoperitoneum
The main consequence of a ruptured cyst is pain and hemoperitoneum. As the great number of ruptured cysts is functional and the hemoperitoneum moderate, conservative treatment is often possible. Pain usually subsides gradually, and the hemoperitoneum disappears. In rare cases, surgery is necessary to stop bleeding. When performing an ultrasound in patients with ruptured cyst, it is a good habit to have a look at the upper abdomen; presence of liquid in the Morrison pouch is indicative of an important bleeding.
A special case is that of patient with coagulation troubles. In these patients, despite severe bleeding, surgeons favor conservative treatment and correction of coagulation disorders before intervention (Fig. 32.6).
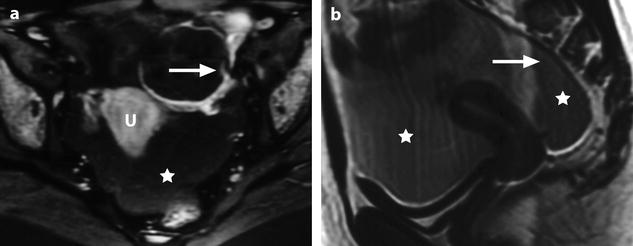

Fig. 32.6
Massive hemorrhage secondary to a ruptured FHC in a 14-year-old girl with myelodysplasia. Ultrasound (Fig. 32.4d) shows a left pelvic cyst with fluid-debris level, but the site of the rupture and the importance of the peritoneal effusion could not be well appreciated. (a) T1-weighted image with gadolinium discloses a cyst with a hypointense content and a wall that enhances markedly. The rupture is well seen (arrow). (b) Sagittal T2-weighted view allows better evaluation of the importance of the hemoperitoneum that contains two fluid levels (arrow points to the more dependant part). Uterus (u); peritoneal effusion (asterisks)
On ultrasound, hemoperitoneum is easily detected when anechoic or slightly echoic. When it is hyperechoic and moderate, it could be missed or underestimated especially by transabdominal ultrasound. When it is hyperechoic and important, it appears as a hyperechoic avascular structure surrounding the uterus on both sides and posteriorly. The two main diagnoses in these cases are ruptured functional cyst and ruptured extrauterine pregnancy. The hyperechoic clotted blood often makes it difficult or impossible to see the normal and/or the diseased ovaries.
On CT scan, the presence of slightly dense ascites in a female patient with sudden pelvic pain should prompt us to look for an ovarian cyst especially a corpus luteum cyst which is the most common cause of rupture (Fig. 32.7).
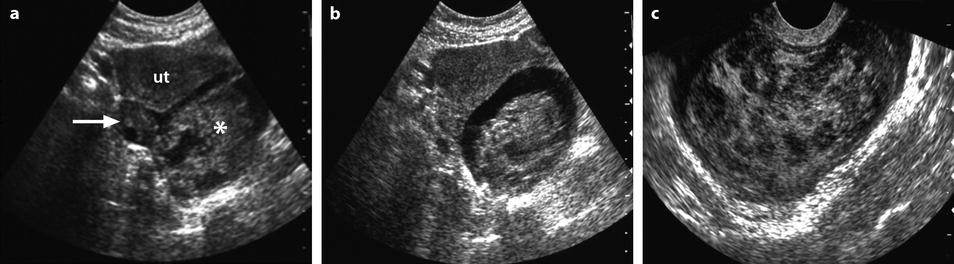
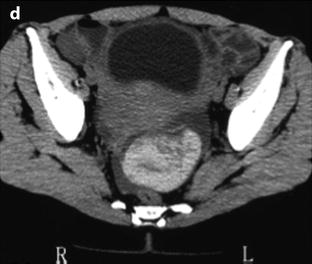


Fig. 32.7
Ruptured FHC on US and CT. (a, b) Transabdominal ultrasound in a young woman with pelvic pain discloses a normal right ovary (horizontal arrow) and a hyperechoic left cyst (asterisk) surrounded by free liquid. Uterus ut. (c) Transvaginal ultrasound better demonstrates the echostructure of the cyst grossly resembling a sponge. The site of the rupture could not be determined. No Doppler flow was detected in the cyst. (d) CT scan without (not shown) and with injection shows the cyst to be spontaneously hyperdense (without contrast uptake) well delineated by free peritoneal fluid. The site of rupture is probably anterior. Note that the peritoneal effusion is denser than the unopacified urine. Surgery confirmed a ruptured FHC
On MRI, the hemoperitoneum may show a wide range of signal depending of the quantity and age of the blood; sedimentation with level(s) may be present (Figs. 32.6 and 32.8).
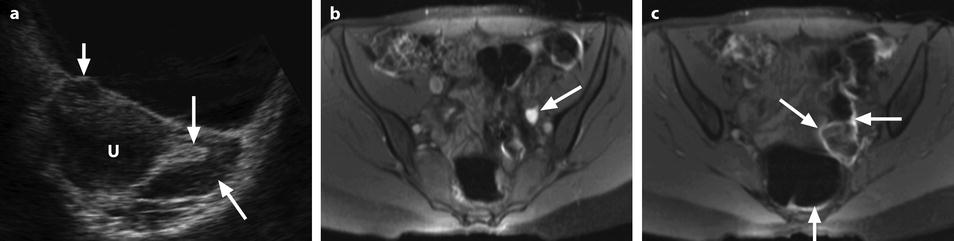

Fig. 32.8
Ruptured corpus luteum with thin T1 hyperintense effusion. (a) Ultrasound in a 16-year-old girl with pain during the second part of her menstrual cycle disclosed a small 10-mm hyperechoic structure (long vertical arrow) of the left ovary (oblique arrow) that merges imperceptibly with adjacent peritoneal fat. The adjacent uterus (u) contains a small subserous leiomyoma (short vertical arrow). (b and c) T1-weighted fat saturation MRI images. At MRI, the ovarian lesion appeared hypointense on T2 (not shown), hyperintense on T1 (not shown), and on T1 fat saturation images (arrow in b). A thin T1 hyperintense effusion related to a small hemoperitoneum is also seen on lower slices (arrows in c). Coelioscopy confirmed the above findings
32.3.3 The Peritoneum
After spontaneous or surgical resolution of the disease, there is usually no consequence on the peritoneum.
However, spontaneous or surgical rupture of an ovarian cancer may prompt peritoneal carcinomatosis. Pseudomyxoma peritonei may be the consequence of a rupture ovarian mucinous carcinoma although it is believed that it arises more frequently from the appendix.
Granulomatous peritonitis may be secondary to a long-standing hemoperitoneum.
32.4 Adnexal Torsion
32.4.1 Physiopathology and Clinical Findings
The clinical and radiological patterns of adnexal torsion are extremely variable. This is due to a complex physiopathology secondary to different factors:
Usually, the torsion involves the ovary and the tube, but it may involve the ovary alone or rarely the tube alone.
The torsion may involve normal adnexa but usually is secondary to a preexisting mass in the adnexa.
The severity of the vascular compression is variable depending on the number of twists and the tightness of the twist(s).
The dual vascular supply of the ovary from the ovarian and uterine arteries.
The intermittent severity of the vascular compression due to the alternation of tightness and untightness of the twist(s) that could result sometimes in complete spontaneous detorsion.
Basically, there are two phases:
(a)
Early phase: venous compression with edema
At this stage, the adnexa are viable. The usual treatment is surgical detorsion either by coelioscopy or laparotomy. If a mass is present, it is removed. Keeping or removing the adnexa will depend on the nature of the associated mass if present and the age of the patient.
(b)
Late phase: compression with hemorrhagic necrosis
The necrosis may be complete or partial. Usually, the surgeon proceeds to detorsion and observes the color of the adnexa. If they are revascularized with only some petechiae, he can keep them. Otherwise, he usually performs adnexectomy or partial resection. The surgical decision will depend also on the nature of the associated mass if present and the age of the patient. Even in case of necrosis, some surgeons advocate keeping the adnexa especially in children or young patients [11–13].
At both the early and late stage, oophoropexy or shortening of the utero-ovarian ligament is sometimes performed ipsilaterally, contralaterally if the ovary has been removed, or bilaterally especially in children [14].
The evolution of torsion is unpredictable. It can rapidly progress to a stage of hemorrhagic necrosis in a few hours or remain at the stage of edema for several days. The intermittent character of the torsion is translated clinically by intermittent pain that in some cases may evolve into a complete spontaneous detorsion with the patient becoming asymptomatic or to completion of the torsion with hemorrhagic necrosis and loss of the adnexa [15].
Clinically, pain secondary to torsion can be confused with that of renal colic, appendicitis, diverticulitis, ruptured or unruptured hemorrhagic functional cyst, ovulation, or any other pelvic pain.
The complex physiopathology is also reflected by a variable radiological appearance. Multitudes of papers have been published concerning adnexal torsion describing a multitude of findings that may confuse the reader. However, it is easy to understand and remember them if they are separated into two groups: findings due to edema in the early phase and findings due to hemorrhagic necrosis observed later. Combination of these two groups is often found. We can also add a third group, the associated findings (Table 32.3).
32.4.2 Early Phase (Edema)
Edema may involve the ovary and the fallopian tube, the ovary alone or the tube alone, depending on which parts of the adnexa are involved by torsion.
32.4.2.1 Edema of the Ovarian Stroma
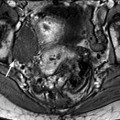
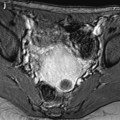
Edema of the ovarian stroma is best appreciated when the torsion involves a normal ovary. It results in an increase in size of the ovary that is sometimes spectacular simulating a mass or tumor. We can calculate either the largest cross-sectional area of the ovary or the ovarian volume to appreciate its increase in size. In our practice, we prefer to use the cross-sectional area because it is easier to calculate and more reproducible. To assess the increase in size of an ovary in polycystic ovary syndrome, the threshold of 6 cm2 is usually used. With regard to adnexal torsion, we set our threshold at 10 cm2 [16]. A torsion is unlikely if the largest ovarian cross-sectional area is <10 cm2 and very likely if it is >10 cm2 in the proper clinical context (Fig. 32.9). Follicles are usually pushed to the periphery by stromal edema.
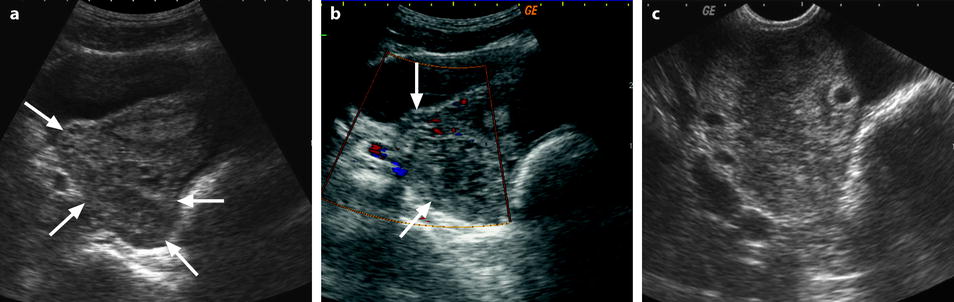
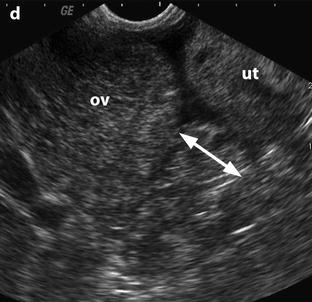


Fig. 32.9
Adnexal torsion without associated mass diagnosed on US. (a) Transabdominal US in a 20-year-old woman complaining of pelvic pain since 36 h displays large right adnexa (arrows) next to a uterus with a thickened endometrium. (b) Close US scanning shows that this enlarged adnexa is composed of two parts: a thickened tube (vertical arrow) and a large ovary (oblique arrow). Some vessels could be detected in the tube by color Doppler. (c) Transrectal ultrasound in this virgin woman clearly identifies a large 5.3 × 4.2 cm (17.8-cm2 cross-sectional area) right ovary with peripheral follicles <1 cm. The ovarian stroma appears partly hyperechoic. (d) Between the ovary (ov) and the uterus (ut), a thickened tube (arrow line), 2.3 cm in width, is seen. A small peritoneal effusion is also present. An arterial flow with a resistivity index of 0.60 could be detected in the ovary. No venous flow could be recorded. Laparoscopy displayed a right adnexal torsion involving the ovary and the tube. The adnexa were viable with some petechiae
When a mass is present in the ovary, we must differentiate between an increase in size due to edema and an increase in size due to the mass. To solve this problem, we calculate what we called the corrected cross-sectional area of the ovary corresponding to the entire cross-sectional area of the ovary minus the cross-sectional area of all follicles, cysts, or masses >1 cm [16]. This is done to be in similar conditions to normal ovaries or polycystic ovaries that only contain follicles <1 cm. After choosing on ultrasound the cross section showing the largest area of ovarian parenchyma, we proceed to the calculation of the corrected cross-sectional area. We also use a threshold of 10 cm2 [16] (Figs. 32.10 and 32.11).
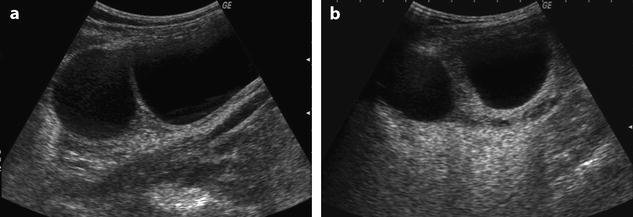
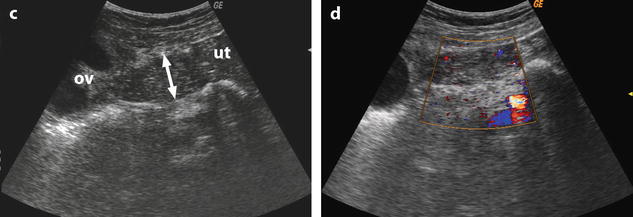
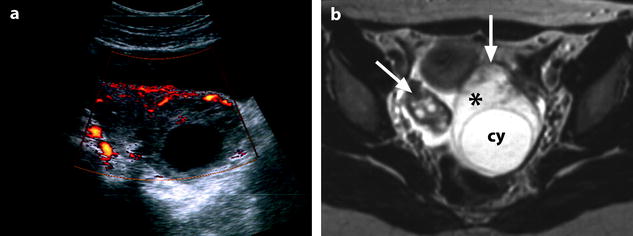


Fig. 32.10
US before and after adnexal torsion in a pregnant patient with follicular cysts. (a) Routine US at 9 weeks and 4 days of amenorrhea shows two right ovarian follicular cysts. The patient was asymptomatic. Notice that the ovarian stroma separating the cysts is not enlarged. (b) At 13 weeks and 1 day of amenorrhea, the patient developed right pelvic pain. Repeated US shows increase in stroma thickness with a corrected cross-sectional area of 13.9 cm2. (c and d) A 19–27-mm thickened tube (arrow line) is seen joining the right ovary (ov) to the uterus (ut). Venous and arterial flow could be recorded in the tube and the ovary. Laparoscopy with detorsion and aspiration of the cysts was performed. The patient completed her pregnancy normally

Fig. 32.11
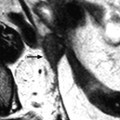
Isolated ovarian torsion with high intensity T2-weighted ovarian edema. A 20-year-old woman with pelvic pain since 24 h. (a) Power Doppler shows a vascularized enlarged ovary containing a 45-mm anechoic cyst (largest ovarian cross-sectional area, 34.11 cm2; largest corrected ovarian cross-sectional area, 17.79 cm2). (b) Axial T2-weighted image (4500/96) shows both ovaries in the same plane. The right ovary (oblique arrow) has a normal-intensity stroma and is surrounded by ascites. On the left, large areas of the ovarian stroma (asterisk) have signal intensity equal to that of the content of the cyst (cy) and to that of ascites. Some areas of the stroma (vertical arrow), the wall of the cyst, and the most external layer of the cortical parenchyma (tunica albuginea) remain hypointense. Laparoscopy confirmed the presence of a left ovarian torsion with a follicular cyst; the left tube was not involved by torsion. The ovary was edematous and viable. Conservative surgery, cystectomy and detorsion, was performed (Figures from Authors’ personal publication: Ghossain et al. [16])
The area(s) needed for computing the cross-sectional or the corrected cross-sectional area can be calculated by drawing it (them) directly on the screen or using the formula: largest diameter (cm) × short diameter (cm) × 0.8 = area (cm2).
Ultrasound
We do not rely on the echogenicity of ovarian parenchyma because it is very subjective and difficult to assess. We compute the cross-sectional area or the corrected cross-sectional area as defined above.
The main difficulty is to differentiate between ovarian parenchyma and an echoic cyst as a hemorrhagic functional cyst, a dermoid cyst, or an endometrioma. This can be particularly difficult by suprapubic ultrasound even for experienced sonographers. Another difficulty is to differentiate between the ovarian parenchyma and an adjacent edematous fallopian tube (Fig. 32.9). At this stage, color flow is usually detected in the ovary and tube (Figs. 32.10 and 32.11). Because of technical limitations, absence of color flow does not mean necessarily that the adnexa are not vascularized especially by transabdominal approach.
CT Scan
On CT scan, the ovary is increased in size and appears hypodense relative to the opposite side (Fig. 32.12). The stromal edema is more difficult to assess than in MRI but can be detected easily if the increase in size is important and if one is aware of this finding (Fig. 32.13). Comparison of pre- and postcontrast images allows differentiating edema from liquid and vascularized from nonvascularized areas and detection of spontaneous hyperdense hemorrhagic necrosis if present (Fig. 32.14).
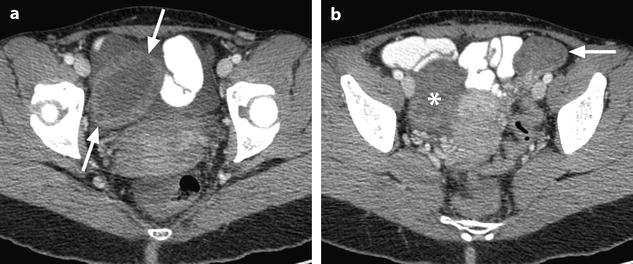
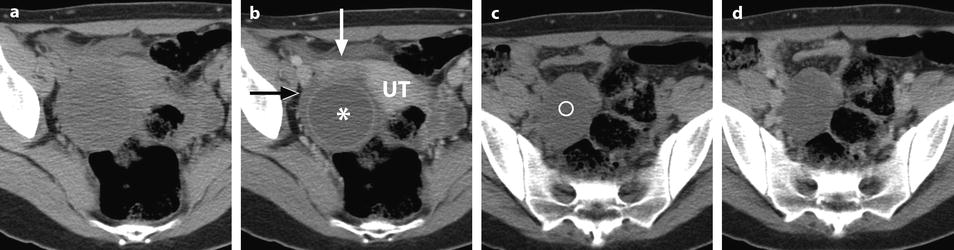
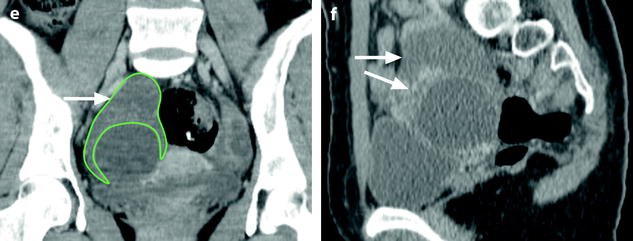
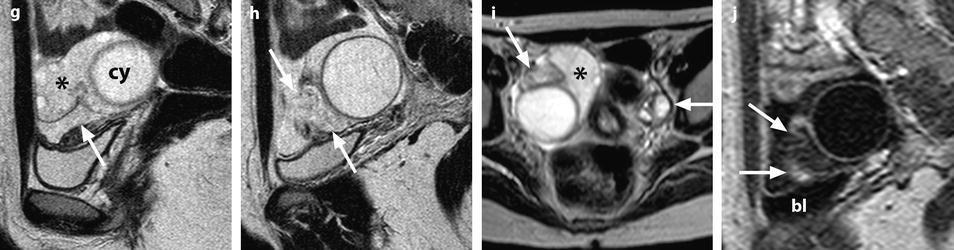
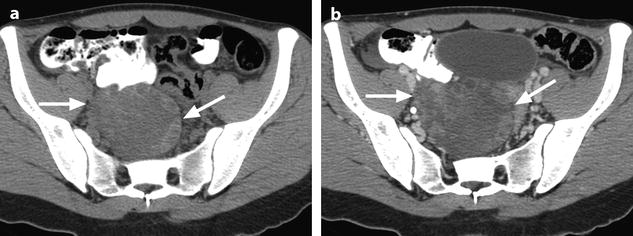
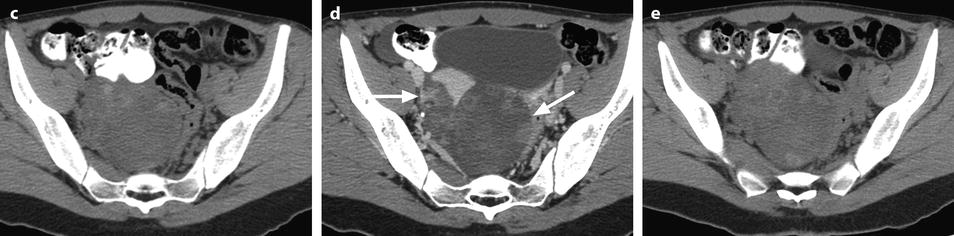
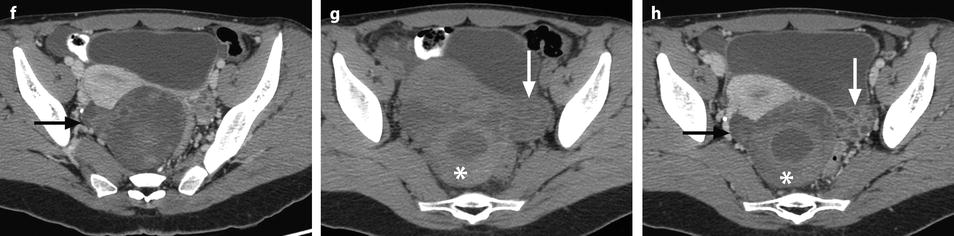
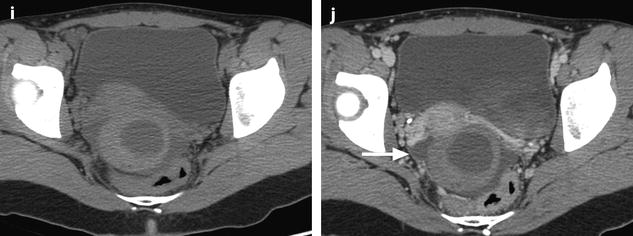

Fig. 32.12
Ovarian torsion without tubal involvement at a viable stage. (a, b) CT scan after contrast injection shows a large right ovary (arrows in a) with 24-cm2 cross section and only small follicles <1 cm without any large mass. The left ovary (arrow in b) is also enlarged due to polycystic disease but not exceeding a cross section of 8.9 cm2. The right ovary is hypodense due to edema with a density in its central portion of 25 HU versus 47 HU for the opposite side. The difference of density is less obvious in the periphery of the ovaries where small follicles are present. Asterisk in b = upper part of the right ovary. No tubal thickening is seen. At surgery, the ovary was twisted but not the tube. It was cyanotic but recovered its normal color after detorsion. No hemorrhagic areas were seen



Fig. 32.13
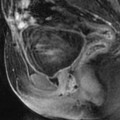
Adnexal torsion at the edematous stage in a 23-year-old woman. CT scans (a) before and (b) after contrast injection display a cyst (asterisk) with an enhancing wall, an enhancing 17-mm thickened tube (vertical arrow) and a slightly enhancing peripheral ovarian parenchyma (horizontal arrow). Uterus = ut. CT scans (c) before and (d) after contrast injection, at a higher level, show more ovarian parenchyma (circle), allowing better density measurement with an enhancement from 23 to 38 HU. (e) Coronal and (f) sagittal reformatting allows to better measure the corrected cross-sectional surface of the ovary (horizontal arrows) that reached (19.3 cm2). In (e) is shown an example of computing the cross-sectional surface of the ovary using direct surface measurement. The thickened tube is also seen in (f) (oblique arrow). (g, h) Sagittal MR T2-weighted images show that the ovarian parenchyma is hyperintense due to edema (asterisk), slightly less than the cystic content of the cyst (cy). The tube is also displayed (arrows) and hyperintense, reaching 32 mm in thickness. (i) Axial T2-weighted image allows comparison between the enlarged right hyperintense ovarian stroma (asterisk) and the small hypointense stroma of the left ovary (horizontal arrow) that can be barely seen between follicles. Right thickened tube = oblique arrow. (j) Dynamic sagittal MRI at a late phase shows serpiginous vessels in the thickened tube (arrows). Bladder bl




Fig. 32.14
Adnexal torsion with partial necrosis. Serial CT scans before injection (a,c,e,g,i) and after injection (b,d,f,h,j), at the same levels. (a, b) At high level, it is difficult to differentiate between the thickened tube (horizontal arrow) and the ovary (oblique arrow). (c–j) At lower levels, the uterus is well visualized after contrast injection, allowing to better distinguish the thickened tube (horizontal white and black arrows) from the right ovary (oblique arrow in d). Left ovary = vertical arrow in g and h. The tube could be followed on serial section from cornua utera to cervix on the right side of the ovary. Contrast enhancement could be demonstrated in the tube but not in the entire ovary. Part of the ovary (asterisk) appeared hyperdense before injection, not enhancing, corresponding to hemorrhagic necrosis. At surgery, the tube was edematous and the ovary partially necrotic. Detorsion and partial ovariectomy were performed
MRI
MRI has two major advantages over ultrasound and/or CT:
(a)
It allows to distinguish clearly between the ovary and adjacent tube and occasionally between the ovarian parenchyma and intraovarian masses, allowing accurately computing the corrected cross-sectional area of the ovary and the thickness of the tube (Figs. 32.13 and 32.15).
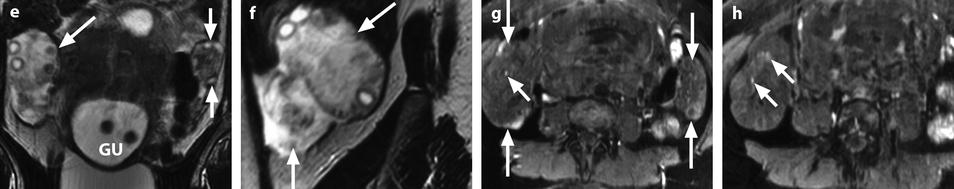


Fig. 32.15
Right adnexal torsion in an 18-week pregnant woman with previous ovarian stimulation. Transabdominal ultrasound in an 18-week pregnant woman complaining of right pelvic pain 1 week after losing one of two fetuses shows both ovaries to be enlarged due to previous ovarian stimulation. However, the right ovary (a) is larger with a 27-cm2 cross-sectional area against 16 cm2 for the left ovary (b). Moreover, color Doppler could not be detected in the right ovary (c), while it was present in the left ovary (d). The diagnosis of right ovarian torsion was made, but the surgeon asked to have more proofs before undergoing surgery in this patient with precious and high-risk pregnancy. MRI without gadolinium injection was performed. (e) The right ovary was enlarged and its stroma hyperintense on T2 (oblique arrow); note some hypointense areas that can be related to normal stroma not still involved by edema or areas where edematous stroma was replaced by necrotic hemorrhage. The left ovary is of smaller size with a normal hypointense stroma on T2 (short vertical arrows). Gravid uterus = GU. (f) Sagittal T2 MRI of the right ovary disclosed not only the large ovary (oblique arrow) but a thickened hyperintense fallopian tube (vertical arrow). (g and h) T1-weighted fat saturation images show both enlarged ovaries (long vertical arrows) and some punctiform hyperintensity hemorrhagic spots of the right ovary (short oblique arrows). At surgery, the right adnexa were twisted twice and appeared blue. After detorsion, the adnexa recovered their normal color and were kept in place after ovarian fixation. Follow-up was uneventful with no recurrent torsion and delivery by cesarean section at 31 weeks and 3 days
(b)
While the echogenicity of the ovarian parenchyma on ultrasound or its density on CT is difficult to appreciate, ovarian parenchyma appears hyperintense on T2 considerably higher than the contralateral side, sometimes confused with liquid (Figs. 32.11 and 32.13). Several clinical cases have been published in the literature where a large edematous ovary has been confused with a cystic mass [17, 18]. The clue to the diagnosis is to demonstrate vascularization in the pseudo-liquid zones either by Doppler or contrast enhancement [16]. Follicular and cyst walls, as well as the most external layer of the cortical parenchyma (tunica albuginea), tended to remain hypointense provided that adequate window settings are used (Fig. 32.11) [16]. It seems also that fibrous tissue is less involved by edema than the normal ovarian parenchyma (Fig. 32.16). In some cases, not all the stroma appears hyperintense on T2. This can be due to areas spared by edema or areas with hemorrhagic necrosis (Figs. 32.11 and 32.15).

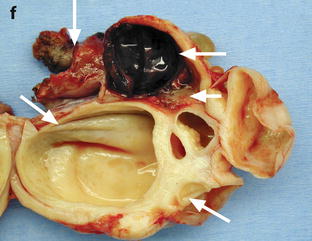



Fig. 32.16
Adnexal torsion with cystadenofibroma and FHC. (a and b) Transabdominal and endovaginal ultrasound in a 40-year-old woman with right pelvic pain discloses a large multicystic right ovary with an echoic structure suggesting an FHC (arrow). The ovary was vascularized on Doppler. (c) T2-, (d) T1- fat saturation, (e) T1-weighted fat saturation gadolinium images, and (f) photography of the surgical specimen disclose: A cystadenofibroma (oblique arrows) with hypointense solid tissue on T2, slightly enhancing after gadolinium injection, corresponding to fibrous white tissue at pathology. An FHC (long horizontal arrow) hyperintense on T2 and hypointense on T1 with a wall that enhances markedly, frankly hemorrhagic on pathology corresponding to the FHC described on ultrasound. An edematous ovarian stroma (short horizontal arrow) hyperintense on T2, enhancing more than the fibrous tissue of the cystadenofibroma. A thickened tube (vertical arrow) hyperintense on T2, enhancing heterogeneously. At surgery, a right adnexal torsion involving the tube and the ovary was present. The right adnexa were edematous and viable. However, because of the presence of a tumor, a right adnexectomy was performed
32.4.2.2 Edema of the Fallopian Tube
Edema of the tube results in tubal thickening that can also be detected on ultrasound, CT, and MRI. A threshold of 15 mm can be proposed [19]. When only the tube is twisted and not the ovary, findings of torsion are only present in the tube and not in the ovary; the leading cause in this condition is a paraovarian cyst or a hydrosalpinx (Figs. 32.17 and 32.18).
Get Clinical Tree app for offline access


Fig. 32.17
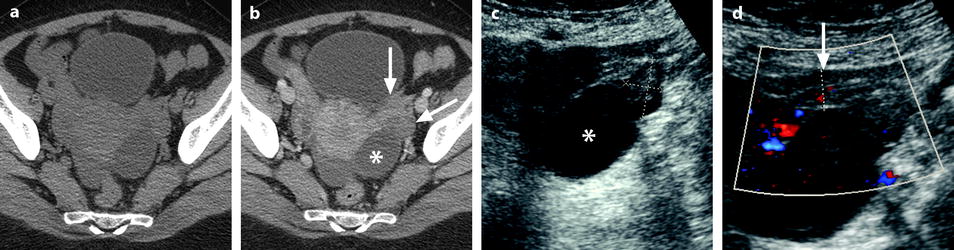
Isolated tubal torsion secondary to paraovarian cyst. (a, b) CT scan before (a) and after (b) contrast injection shows an enlarged vascularized left adnexa but with difficulty to differentiate between a cyst (asterisk), the left ovary (oblique arrow), and the left tube (vertical arrow




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree