Congenital diseases
Adrenal cyst unassociated with malignancy
Hyperplasia
Trauma
Hemorrhage
Infection
Normal Anatomy
The right adrenal gland is typically triangular, with an average volume of 5.4 cm3, and the left is typically crescent-shaped, with an average volume of 5 cm3. Adrenal glands are located at the superomedial pole of each kidney. They are enveloped by the perirenal fascia along with the kidney on each side.
Each gland consists of three portions – an anteromedial ridge, flanked by medial and lateral limbs. Each gland lies in close proximity to the diaphragmatic crus, and the lateral limb of the right gland lies adjacent to the posteromedial aspect of the liver (Fig. 10.1a–c).
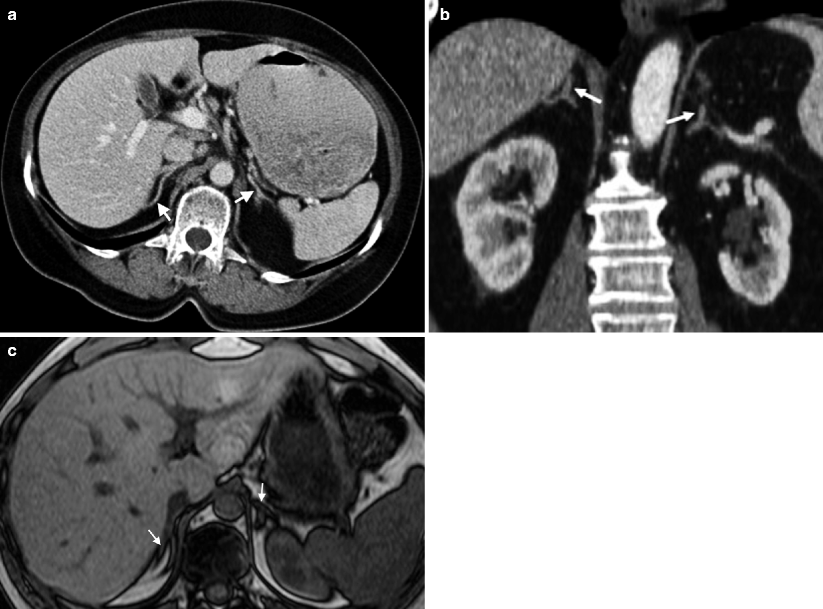
Fig. 10.1
Normal adrenal gland. Axial (a) and coronal (b) unenhanced CT images demonstrate bilateral normal adrenals with V-shape appearance (arrows). (c) Axial T1-weighted image reveals bilateral V-shaped adrenal glands (arrows) isointense to the liver parenchyma
In cases of renal agenesis, the shape of the adrenal gland is abnormal. It is usually elongated and is often mistaken for a diaphragmatic crus (Fig. 10.2a, b).
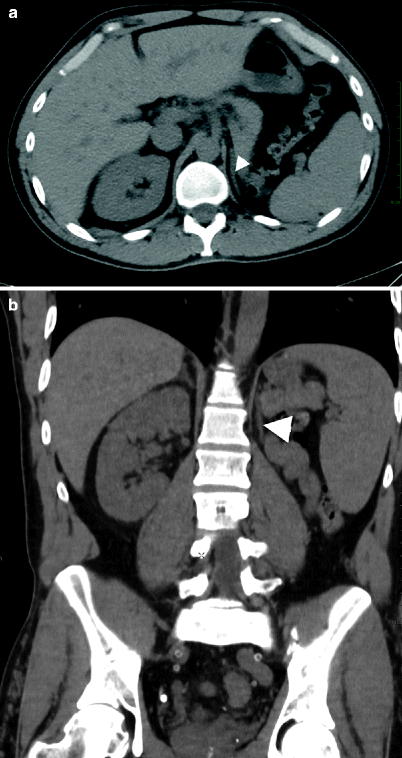
Fig. 10.2
Abnormal adrenal gland shape. Axial (a) and coronal (b) CT images of a patient with renal agenesis reveal elongated shape of the left adrenal gland (arrowhead)
Congenital Diseases
(a)
Embryologic Abnormalities
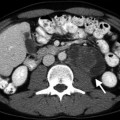
Adrenal agenesis is a rare congenital abnormality that usually occurs in a setting of ipsilateral renal agenesis (Fig. 10.3). Ten percent of cases of renal agenesis also exhibit adrenal agenesis. Adrenal hypoplasia is associated with triploidy, pituitary and central nervous system (CNS) disorders due to adrenocorticotropic hormone (ACTH) deficiency, adrenoleukodystrophy, and Zellweger’s syndrome. Horseshoe adrenal refers to a single midline mass of adrenal tissue which is generally located posterior to the aorta and anterior to the inferior vena cava (IVC). It is associated with asplenia.
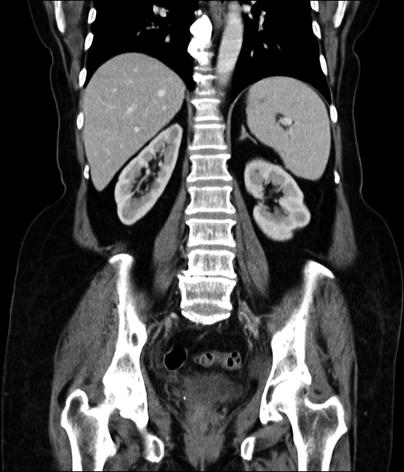

Fig. 10.3
Adrenal agenesis. Contrast-enhanced coronal CT demonstrates absence of right adrenal gland secondary to adrenal agenesis
(b)
Congenital Adrenal Hyperplasia (CAH)
CAH is an autosomal recessive disorder characterized by deficient production of cortisol. Ninety-five percent of patients with CAH have 21-hydroxylase deficiency. Precocious puberty in boys and virilization in girls constitute the most frequent presenting symptoms. Adrenal gland enlargement resulting from hyperplasia can be visualized on imaging studies and may be either diffuse or nodular enlargement, usually with preservation of the normal adrenal shape. Enlargement of heterotopic adrenal tissue in other organs such as testes in males may be observed (Fig. 10.4a–e) (see also the discussion of “TTAGS” in Chap. 6).
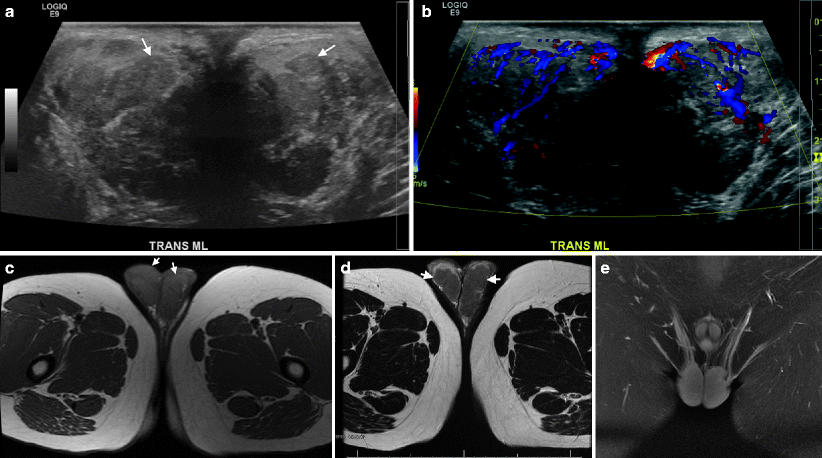

Fig. 10.4
Heterotopic adrenal tissue, forming tumoral masses in the testes of a patient with poorly controlled adrenogenital syndrome. These masses are designated “testicular tumors of the adrenogenital syndrome,” or “TTAGS.” (a) Grayscale ultrasound demonstrates bilateral enlarged testis with heterogeneous echotexture and multiple hypoechoic masses (arrows) replacing the normal testicular parenchyma. (b) Color Doppler image demonstrates increased vascularity of the testis with no mass effect on the vessels. Axial T1 (c) and T2-weighted (d) images reveal replacement of testicular parenchyma with low-intensity lesions (arrows). (e) Contrast-enhanced T1W study demonstrates diffuse enhancement of the testes
(c)
Wolman’s Disease
Deficiency of lysosomal acid lipase results in Wolman’s disease, which is a fatal autosomal recessive condition. Unmetabolized cholesterol esters and triglycerides are abnormally stored in the adrenal glands and other viscera. Presenting signs and symptoms include hepatosplenomegaly, abdominal distension, steatorrhea, vomiting, hypotonia, and failure to thrive. CT findings in Wolman’s disease include a hypodense enlarged liver, splenomegaly, and bilateral symmetrically enlarged adrenal glands with punctate calcifications.
(d)
Beckwith-Wiedemann Syndrome
Beckwith-Wiedemann syndrome encompasses a spectrum of anomalies, including omphalocele, overgrowth abnormalities such as macroglossia and hemihypertrophy, enlargement of various organs, as well as cysts and solid tumors of various organs, including benign adrenal cysts and adrenocortical carcinomas.
Adrenal Cysts
General Information
Adrenal cysts are uncommon but account for nearly 5 % of incidentally detected adrenal lesions.
Although adrenal cysts are frequently asymptomatic, patients may occasionally present with complaints of flank pain, nausea, or vomiting, resulting mostly from local compression effect.
Only 7 % of adrenal cysts are associated with malignancy.
Based on the histopathological evaluation, adrenal cysts are subdivided into four types, namely, endothelial cyst, pseudocyst, parasitic cyst, and epithelial cyst.
Endothelial cysts, which are the most common pathologic subtype, include lymphangiomatous and hemangiomatous cysts and account for a significant proportion of adrenal cysts.
Adrenal pseudocysts constitute 39–42 % of adrenal cysts. Pseudocysts do not have a true epithelial lining, are unilateral, and are usually seen as sequelae of prior hemorrhage.
Parasitic cysts, usually caused by Echinococcus granulosus, are quite rare. Similar to those in the other organs, adrenal hydatid cysts can be detected with ultrasound, CT, and MRI. The imaging features depend on the stage of the evolution of the disease, and the detection of daughter cysts by CT and MRI is characteristic.
Imaging
Plain-Film Radiography
Infrequently, curvilinear or punctate calcifications can be detected by plain-film radiographs.
Ultrasound
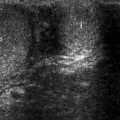
Adrenal cysts appear on ultrasound as well-defined round-oval-shaped anechoic structures with posterior acoustic enhancement. Hyperechoic pattern may result from hemorrhage in the cyst.
Mass effect on surrounding structures may be encountered in large adrenal cysts.
Intracystic hemorrhage may mimic an adrenal mass.
Computed Tomography
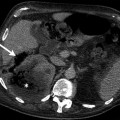
True cysts characteristically have fluid attenuation, usually lower than 20 Hounsfield unit (HU) and smooth borders with thin, non-enhancing walls on CT (Fig. 10.5a, b).
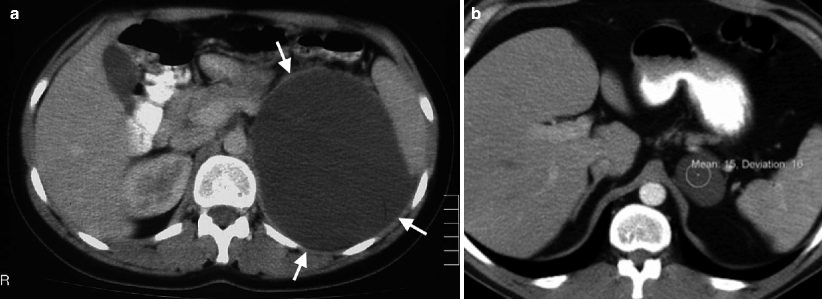
Fig. 10.5
Adrenal cyst. (a) Axial contrast-enhanced CT demonstrates a well-defined large cystic adrenal lesion (arrows) with low attenuation. (b) Axial contrast-enhanced CT of another patient reveals an adrenal cyst. The attenuation value of the lesion is 15 HU (Hounsfield unit)
Calcifications can be seen in more than half of adrenal cysts on CT.
Lack of contrast enhancement on CT favors a diagnosis of adrenal cyst.
Magnetic Resonance Imaging
MRI may be more helpful for a specific diagnosis in cases where the internal density increases due to hemorrhage or infection.
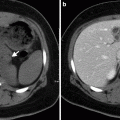
Characteristically, simple cysts are hypointense on T1-weighted and hyperintense on T2-weighted MRI images, without any soft tissue component or any internal enhancement (Fig. 10.6a, b).
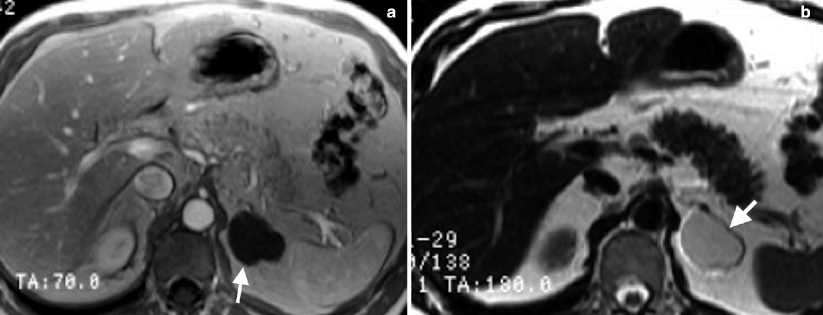
Fig. 10.6
Adrenal cyst. (a) Axial T1-weighted image reveals hypointense well-defined cyst (arrow). (b) Cystic lesion (arrow) appears hyperintense on T2-weighted image
Hemorrhage tends to appear hyperintense on both T1- and T2-weighted MRI.
Pathology
Adrenal cyst is usually discovered incidentally; it is rare for one to become large enough to be symptomatic. About 85 % of adrenal cysts probably result from hemorrhage in normal or abnormal adrenals, some with vascular malformations, and are designated “pseudocysts” or “endothelial cysts,” depending upon whether an endothelial lining is present (Figs. 10.7 and 10.8).
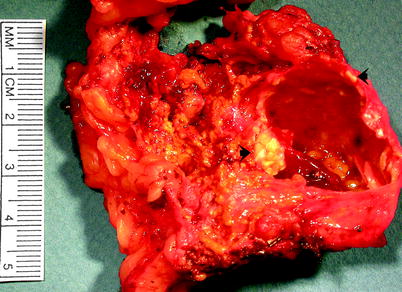
Fig. 10.7
Adrenal cyst. This was a pseudocyst; it lacked any identifiable lining cells. Pseudocysts vary in size. They are usually unilocular and often contain hemorrhagic fluid of variable color and consistency. Dystrophic calcification may be present in the cyst wall, as shown by the arrows (Image courtesy of Stacy Kim, MD)
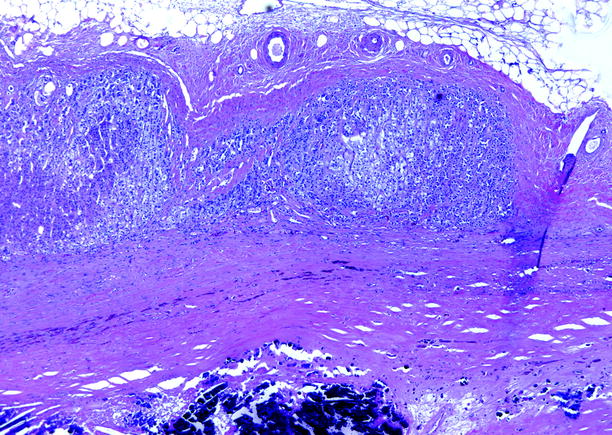
Fig. 10.8
Adrenal cyst. Adipose and normal adrenal tissues are present in the upper half of the image. The lower half of the image is the fibrous wall of a cystic space that contained amorphous material; extensive calcification in the wall is present at the bottom
Rarely, adrenal cysts are parasitic in origin, usually secondary to echinococcus infestation. About 9 % of adrenal cysts have an epithelial lining, likely representing cystic change in entrapped mesothelium or in heterotopic urogenital tissue; others represent cystic change within adrenal neoplasms, including cortical adenoma, cortical carcinoma, or pheochromocytoma.
Adrenal Cortical Hyperplasia
General Information
Adrenal cortical hyperplasia results from a variety of influences and takes a variety of forms (see section on Pathology).
Imaging
Computed Tomography
Adrenal hyperplasia can be excluded if the adrenal limb width is <3 mm.
A width value of adrenal limb >5 mm suggests adrenal hyperplasia.
In some cases, the glands may be diffusely enlarged without any distinct nodule.
In some cases, the glands show diffuse enlargement accompanied by nodules of hyperplastic adrenal cortical tissue.
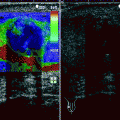
In cases of macronodular hyperplasia with marked adrenal enlargement (MHMAE), CT reveals massively enlarged adrenal glands, with multiple macronodules with soft tissue density measuring up to 5 cm as well as the micronodules (Fig. 10.9a).
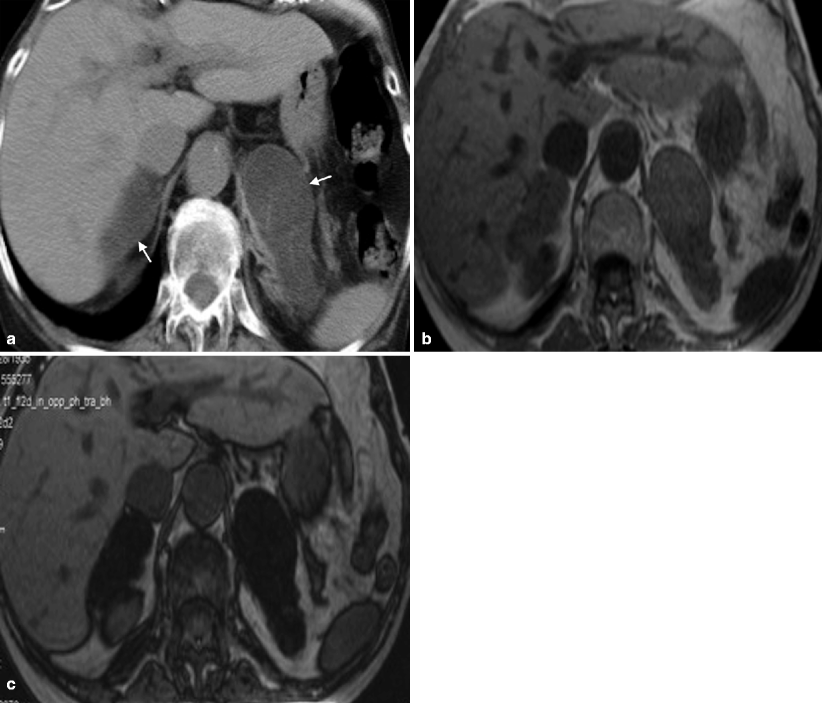
Fig. 10.9
Macronodular hyperplasia with marked adrenal enlargement (MHMAE). (a) Axial unenhanced CT demonstrates bilateral large low-attenuation adrenal masses (arrows). Adrenal lesions appear isointense to liver parenchyma on in-phase image (b) and demonstrates signal loss on out-of-phase sequence (c)
Magnetic Resonance Imaging
MHMAE appears hypointense on T1-weighted images compared to the liver and isointense relative to muscle.
It is hyperintense on T2-weighted images compared to liver.
MHMAE on in-out-phase imaging usually demonstrates drop in the signal on out-of-phase imaging due to chemical shift from high intrinsic lipid content similar to adenoma (Fig. 10.9b, c).
Pathology
Adrenal Cortical Hyperplasia: Diffuse and Nodular
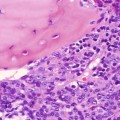
Cortical hyperplasia implies a nonneoplastic increase in the number of cortical cells (Figs. 10.10 and 10.11). It is usually bilateral; may be diffuse, nodular, or both; and may be accompanied by features of eucorticalism, hypercortisolism, hyperaldosteronism, or virilization. The majority of patients with cortical nodularity have normal adrenal function.
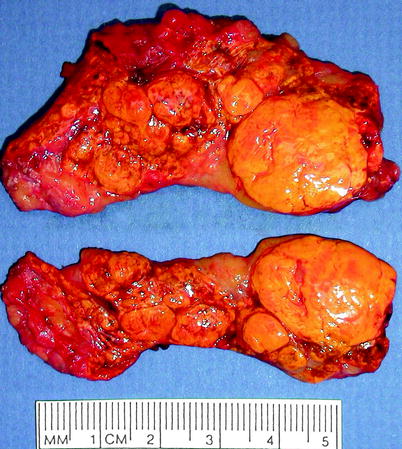
Fig. 10.10
Adrenal cortical hyperplasia, diffuse and nodular. Nodular adrenal glands are composed of multiple (and usually bilateral) discrete or confluent nodules that can be greater than 2.0 cm in diameter. Nodules are commonly present in the surrounding fat. The nodules mainly consist only of cortical tissue, but some show fibrosis, cyst formation, fatty change, myeloid metaplasia, or, rarely, osseous metaplastic change
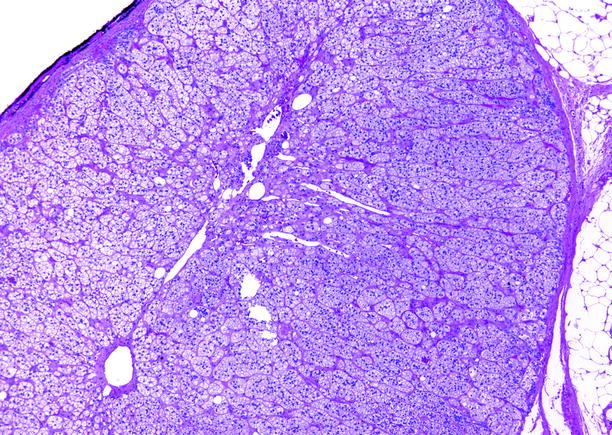
Fig. 10.11
Adrenal cortical hyperplasia, diffuse and nodular. The zona fasciculata is markedly expanded. No medullary tissue is present in this section
Cortical nodularity is commonly observed at surgery and at autopsy: multiple nodules are noted in 1.5–2.9 % of autopsies. Its etiology may be related to localized cortical ischemia with secondary regeneration and hyperplasia. Aging, hypertension, and diabetes mellitus are associated with increased incidence of nodularity.
Macronodular Hyperplasia with Marked Adrenal Enlargement (MHMAE)
This uncommon condition is characterized clinically by autonomous hypercortisolism (independent of pituitary or extrapituitary ACTH stimulation), resulting from aberrant expression of hormone receptors in adrenal cortical cells.
In this condition, both adrenals are greatly enlarged: combined gland weights typically range from 60 to 180 g (Fig. 10.12).
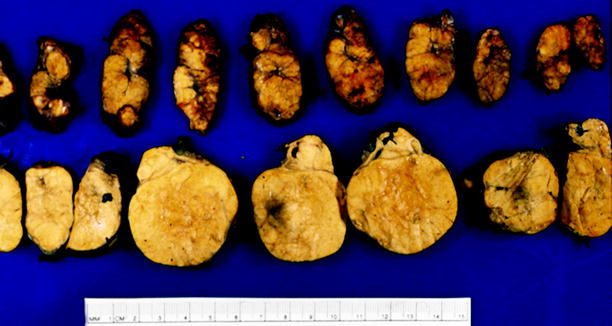
Fig. 10.12
Macronodular hyperplasia with marked adrenal enlargement. The smaller adrenal, sectioned at top, weighed 39 g, and the larger adrenal, sectioned at bottom, weighed 79 g. The adrenal cortex consists of innumerable variably sized hyperplastic nodules (From MacLennan and Cheng (2011), with permission)
Primary Pigmented Nodular Adrenal Cortical Disease (PPNAD)
This disease is of unknown etiology and pathogenesis. It is characterized clinically by autonomous hypercortisolism (independent of pituitary or extrapituitary ACTH stimulation), typically occurring in young persons, most often females.
PPNAD is found in 45 % of cases of Carney’s complex. Normal in size, the adrenals contain multiple nodules, including macronodules, and have an average weight of 9.6 g.
The adrenals may be difficult to remove as the nodules are not encapsulated and may extend beyond the capsule into fat. Nodules range in color (gray to brown to black) and shape but are generally located within the zona reticularis and/or at the cortical-medullary junction (Figs. 10.13 and 10.14).
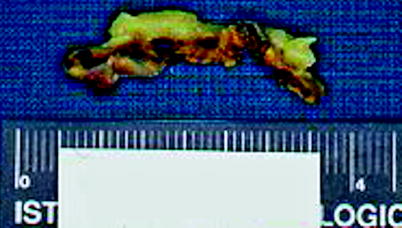
Fig. 10.13
Primary pigmented nodular adrenal cortical disease (PPNAD). Adrenal from a 27-year-old female with hypercortisolism and other features consistent with Carney’s complex. The left adrenal gland was removed on the basis of being radiologically abnormal; it weighed 4.8 g and contained multiple dark brown nodules (From MacLennan and Cheng (2011), with permission)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree