Diffuse prostatic enlargement
Adenomatous nodule(s) within the TZ
Variable TZ echogenicity
Bulging of the prostate capsule
Cystic changes and/or calcifications in the TZ
Marked thinning of the PZ
Increased arterial flow velocities and RI values
Pronounced distinction of the surgical capsule
Hydroureteronephrosis
Bladder trabeculation or diverticulation
Elevation of the bladder base
Increased PVR
BPH presents either as diffuse prostatic enlargement or, in some cases, as discrete single or multiple nodules in the TZ or PGZ.
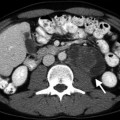
Diffuse expansion of the TZ by BPH is usually visualized as low-echo areas and can easily be distinguished from the PZ, which exhibits higher echogenicity (Fig. 2.1).
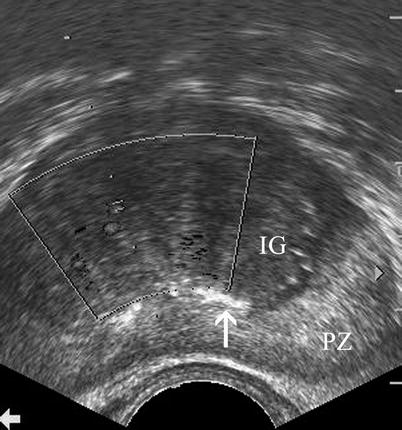

Fig. 2.1
Benign prostatic hyperplasia. Transverse TRUS image reveals enlargement of the TZ with low echo compared with the PZ with higher echogenicity (IG inner gland (TZ), PZ peripheral zone). Note that the surgical capsule is partially demarcated by corpora amylacea (arrow)
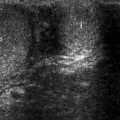
Discrete BPH nodules have variable ultrasound appearances: they may exhibit hyperechoic, hypoechoic, isoechoic, or mixed acoustic properties (Fig. 2.2). They may be surrounded by a hypoechoic rim and may cause bulging of the prostatic capsule without any disruption of the capsule or periprostatic adipose tissue.
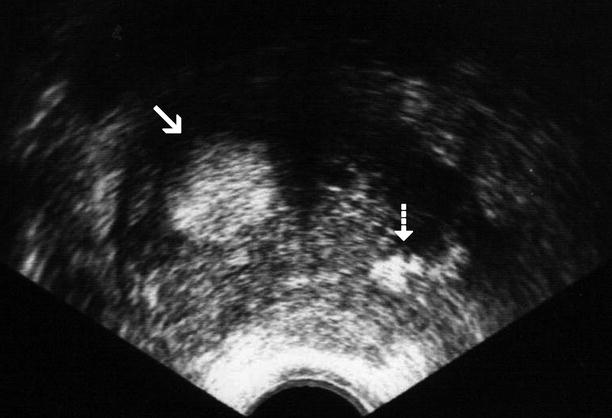

Fig. 2.2
Nodular benign prostatic hyperplasia. Transverse TRUS image demonstrates an enlarged TZ with a hyperechoic nodule in the right lobe (arrow) as well as corpora amylacea (dashed arrow) in the left lobe
In patients with BPH, cystic changes and calcifications may be detected in the TZ (Fig. 2.3). The PZ may be compressed to a few millimeters in thickness when BPH develops.
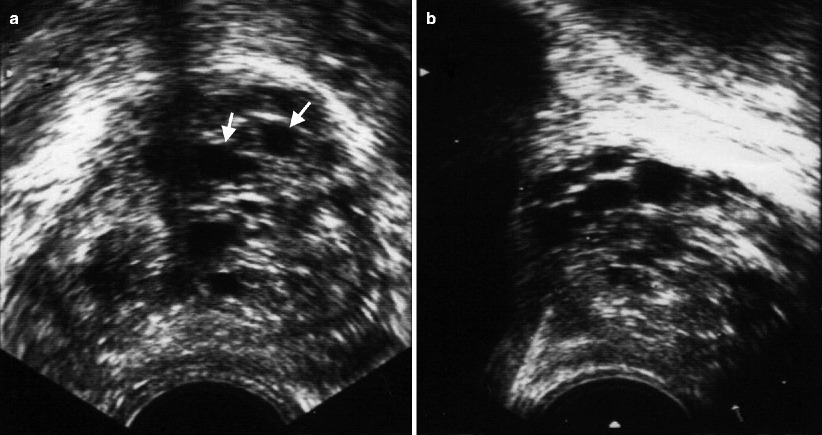

Fig. 2.3
Benign prostatic hyperplasia. Transverse (a) and longitudinal (b) TRUS images reveal cystic changes (arrows) associated with hyperplasia of TZ
A “surgical capsule” separates the enlarged TZ from the PZ and is identifiable on ultrasound as a well-defined change in echogenicity or a hypoechoic rim around the TZ with or without concurrent calcifications representing corpora amylacea.
Increased arterial flow velocities and relatively higher RI values can be measured in BPH, as compared to similar values measured in prostate cancer and normal prostate tissue, though significant overlap occurs.
Digital rectal examination (DRE) is an inaccurate means of estimating prostate size. TRUS can reliably measure the prostate volume. The technique involves the use of an ellipsoid formula: the three largest diameters (width × height × length) are multiplied by the factor 0.5. Volume can be converted to weight as 1 ml of prostate tissue weighs approximately 1 g. In men older than 50 years of age, a prostate gland weighing more than 40 g is considered to be significantly enlarged.
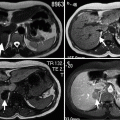
Another indication for ultrasound is the evaluation of prostatic anatomy in patients with a prior transurethral prostatic for BPH, with a view to assessing defects in the central part of the TZ superior to verumontanum related to prior resections as well as detecting residual or recurrent hyperplastic nodules (Fig. 2.4a, b).
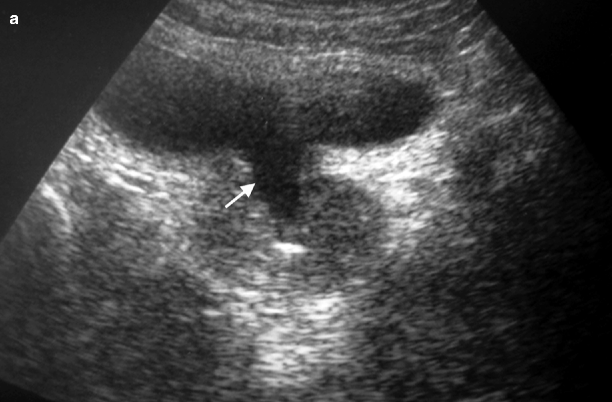
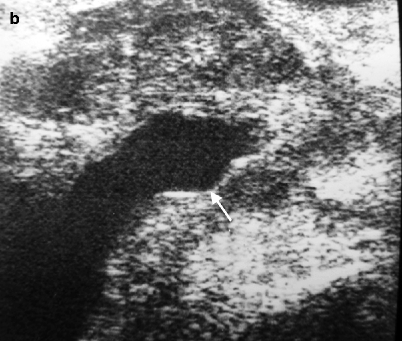


Fig. 2.4
Surgical defect in prostate. Transabdominal transverse (a) and longitudinal (b) TRUS images reveal a central defect (arrows) superior to the verumontanum as a result of transurethral resection
Computed Tomography
Increased attenuation or enhancement of the TZ as well as enlargement of the TZ and the whole prostate gland may be appreciated on computed tomography (CT), though it plays no significant role in the evaluation of patients with BPH.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) can demonstrate features of BPH, namely, enlarged transitional zone.
On T2-weighted MRI, predominantly stromal-type BPH, which tends to be hypointense and lacks nodularity, can be distinguished from predominantly glandular-type BPH, which tends to be hyperintense and nodular.
BPH exhibits a wide spectrum of low to high signal intensity, depending on the stromal versus glandular tissue content (Fig. 2.5).
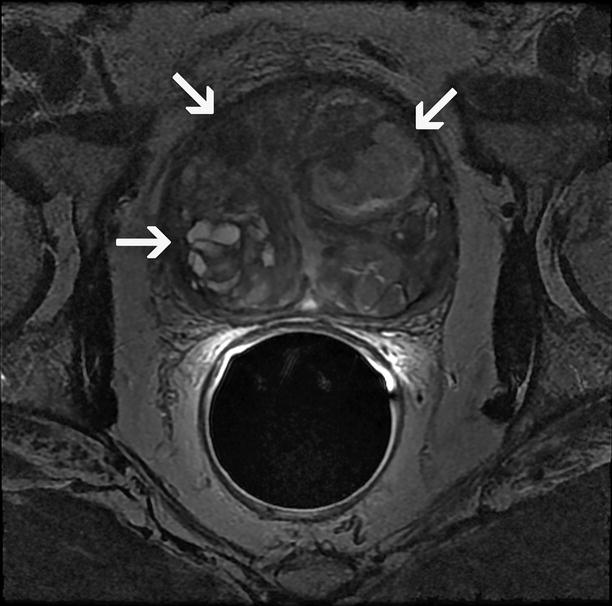
Fig. 2.5
Nodular benign prostatic hyperplasia. Axial T2-weighted MRI demonstrates adenomatous nodules (arrows) with variable signal intensity located in the TZ in a patient with BPH
MRI enables the assessment of TZ/PZ volume ratio and the extent of prostatic protrusion into the urinary bladder (Fig. 2.6a, b).
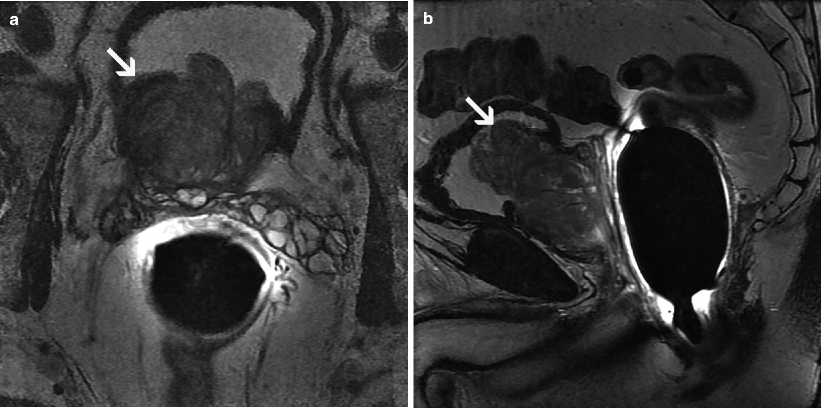
Fig. 2.6
Benign prostatic hyperplasia involving the transition zone. Axial (a) and sagittal (b) T2-weighted MR images demonstrate protrusion of the hyperplastic prostate tissue into the vesical cavity (arrow)
On MRS, higher metabolic ratio of choline + creatine/citrate (CC/Ci) and choline/creatine (Cho/Cr) ratios can be detected in PC as compared to the same measurements in BPH (Fig. 2.7).
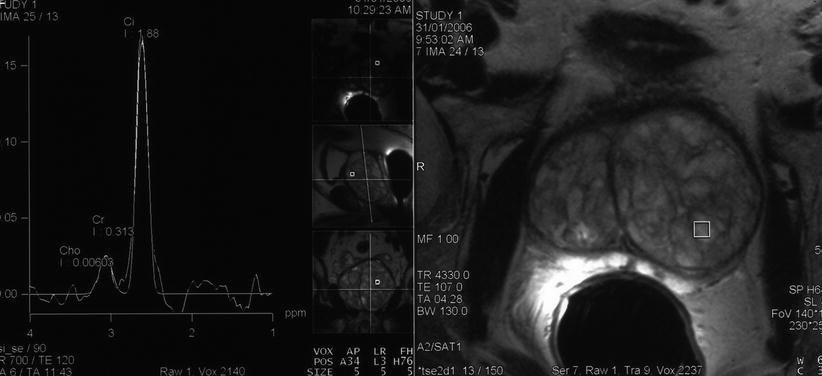
Fig. 2.7
Spectroscopic evaluation of nodular benign prostatic hyperplasia. MRI spectroscopic signature obtained from a nodule of BPH demonstrates citrate (Ci) dominance and no abnormal elevation of choline (Ch) or creatinine (Cr)
DWI of a BPH nodule reveals a nonhomogeneous and lower signal intensity compared with the normal PZ.
Pathology
Enlargement of the prostate due to benign prostatic hyperplasia (BPH) is a consequence of overgrowth of the epithelium and fibromuscular tissue of the transition zone and periurethral area of the prostate.
In the simplest sense, stromal cells convert circulating testosterone to dihydrotestosterone, which in turn induces growth factors that stimulate stromal cell proliferation and reduce the death rate of epithelial cells.
There is no definitive evidence of increased proliferation of epithelial cells.
Rather, the overall increase in number of epithelial and stromal cells that characterizes BPH is thought to result predominantly from a reduction in the rate of cell death of the epithelial component, with the result that senescent cells accumulate in the prostate.
The slowly developing, progressive, and long-standing bladder outlet obstruction results in compensatory changes in the bladder, including detrusor muscle hypertrophy with trabeculation, and the formation of small to large diverticula.
Stone formation, chronic infection, detrusor muscle decompensation, and even upper tract deterioration may eventually occur in cases of unrelieved obstruction.
Most hyperplastic prostates weigh between 60 and 100 g but can be much larger (Fig. 2.8).
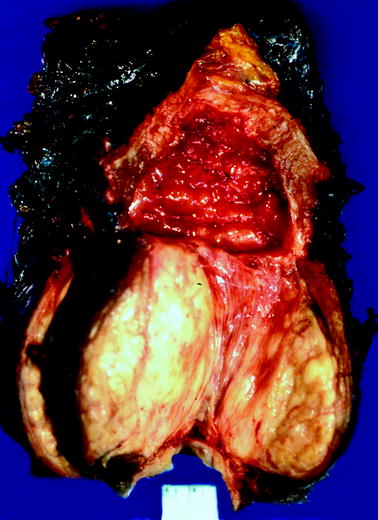
Fig. 2.8
Benign prostatic hyperplasia. The patient underwent cystoprostatectomy for urothelial carcinoma. Bladder cavity is at top. The prostate is markedly enlarged and distorted by prostatic hyperplasia. The bladder wall is markedly thickened, and bladder trabeculation is evident; these findings denote long-standing bladder outlet obstruction
The nodules of BPH are variably sized, soft or firm, rubbery, and yellow gray and bulge from the cut surface when transected.
Although BPH is not a precursor of cancer, most prostate cancers arise in patients with some degree of concomitant nodular hyperplasia (Fig. 2.9), and cancer is found incidentally in a significant number (10 %) of transurethral prostatectomy specimens.
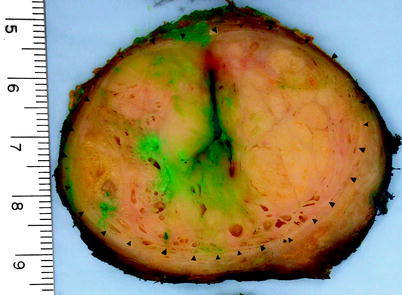
Fig. 2.9
Benign prostatic hyperplasia. Cross section of a radical prostatectomy specimen from a patient who had both prostate cancer and benign prostatic hyperplasia; the prostate gland weighed 98 g. The arrowheads outline the nodular expansile bulging hyperplastic prostate tissue in the transition zone, which compresses the adjacent non-hyperplastic tissue
Calculi are sometimes present (Fig. 2.10), and zones of infarction due to vascular insufficiency are seen in about 20 % of cases (Fig. 2.11).
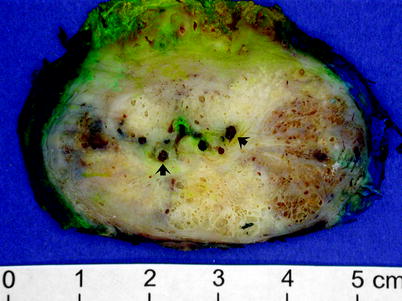
Fig. 2.10
Benign prostatic hyperplasia. Cross section of a radical prostatectomy specimen from a patient who had both prostate cancer and benign prostatic hyperplasia. The arrows indicate prostatic calculi, which often accompany benign prostatic hyperplasia (Image courtesy of Lisa Peck)
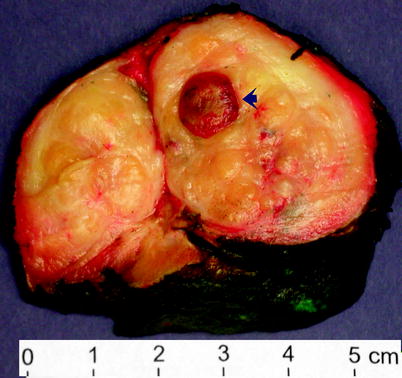
Fig. 2.11
Benign prostatic hyperplasia. Cross section of a markedly enlarged prostate in another patient who underwent cystoprostatectomy for urothelial carcinoma. The prostate is distorted by bulging nodules of hyperplastic prostate tissue. The arrow indicates a sharply circumscribed prostatic infarct, a finding that is relatively common in BPH
BPH typically involves the inner aspect of the prostate (the transition zone), but nodule formation is often also present in the periurethral tissue at the bladder neck.
Pronounced enlargement of this so-called median bar may create a nodule that protrudes into the bladder lumen (Fig. 2.12).
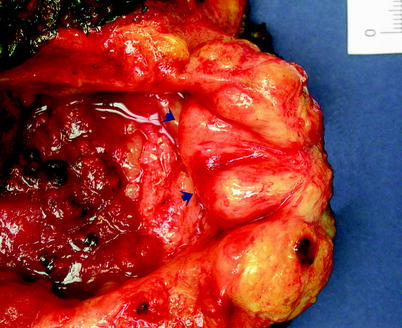
Fig. 2.12
Benign prostatic hyperplasia. Arrows indicate hyperplastic prostate tissue that has developed at the region of the bladder neck and is protruding into the vesical cavity in this cystoprostatectomy specimen from a patient with extensive urothelial carcinoma
Microscopically, BPH consists of nodules composed of varying proportions of hyperplastic epithelium and stromal cells, including fibroblasts, smooth muscle cells, and undifferentiated mesenchymal cells.
Nodules are commonly predominantly epithelial, predominantly stromal, or mixtures of these elements (Figs. 2.13 and 2.14).
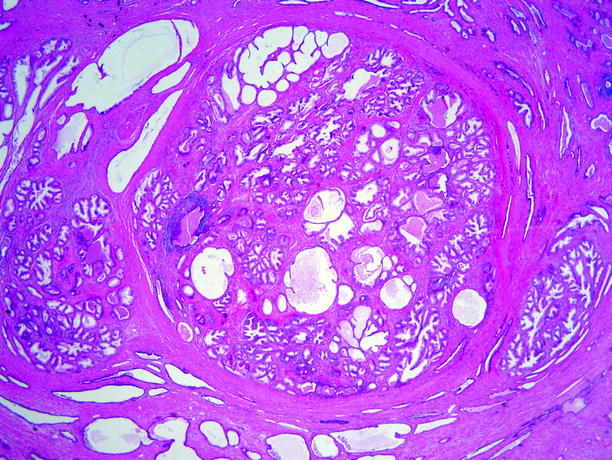
Fig. 2.13
Benign prostatic hyperplasia. This very low-power microscopic view illustrates the nodularity of the process: three fairly well-circumscribed expansile nodules of BPH, of varying size, are present

Fig. 2.14
Benign prostatic hyperplasia. In this low-power view of a case with BPH, the hyperplasia is almost entirely stromal; very few glands are present. This is in marked contrast to the findings in Fig. 2.13, in which the hyperplasia is almost entirely glandular (From MacLennan et al. (2003), with permission)
Predominantly epithelial nodules are composed of branching and converging duct-acinar elements. The glands are typically medium to large and sometimes cystic.
The epithelium is proliferative, with complex architecture and often papillary infoldings.
The epithelium consists of two distinct layers of cells: a layer of small, dark, elongated, cuboidal to oval basal cells and, overlying them, a layer of tall columnar secretory cells with abundant clear to lightly eosinophilic or sometimes granular cytoplasm (Fig. 2.15).
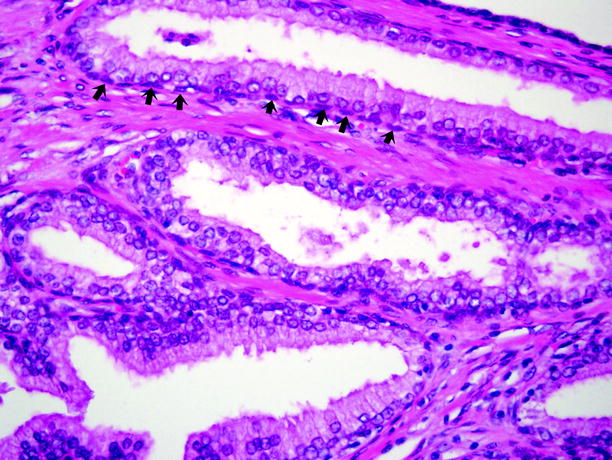
Fig. 2.15
Benign prostatic hyperplasia. The glands of BPH are lined by two cell layers: a layer of tall columnar secretory cells sits atop a layer of flattened and relatively inconspicuous basal cells, which are indicated by arrows. Basal cells are often difficult to discern with routine histologic staining, but they can be highlighted readily by high molecular weight cytokeratin immunostains
Cell nuclei are usually well aligned and uniform, with inconspicuous nucleoli and lacking mitotic activity.
Differential Diagnosis
A major limitation of TRUS is the low specificity of a nodular hypoechoic lesion.
Apart from prostate adenocarcinoma, a number of benign entities such as atrophy, infarction, focal prostatitis, and prostatic cyst may have a similar sonographic appearance.
Typically, PC occurs in PZ and BPH involves the TZ.
A hypoechoic nodule of BPH may occasionally simulate PC because it may appear to be located in the PZ, even though it actually lies in the TZ.
Asymmetry in the PZ size, capsular distortion, and loss of the echogenicity difference between the TZ and the PZ are TRUS features suggesting cancer rather than BPH.
A hypoechoic nodule in the TZ represents BPH in more than 80 % of cases.
MRI findings such as ill-defined margins, lack of capsule, lenticular shape, and invasion of the anterior fibromuscular stroma are suspicious for the presence of TZ cancer.
BPH nodules typically have well-defined margins, visible capsules, and a round shape. They tend to displace the anterior fibromuscular stroma rather than invading it.
Scar tissue and calcifications appear hypointense on T2-weighted images.
TRUS-guided biopsy is the only means for a definitive diagnosis of PC.
Pearls and Pitfalls
BPH that causes elevation of the bladder base may be misinterpreted as a mass lesion originating from the bladder base. Sagittal TRUS images or multiplanar evaluation by MRI may be helpful in making this distinction.
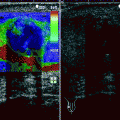
PC frequently coexists with BPH. Infrequently, BPH nodules with increased blood flow at CDUS are present in the PZ.
On dynamic contrast-enhanced MRI, PC, hyperplastic nodules, and prostatitis all demonstrate increased enhancement and consequently are difficult to distinguish from one another.
A previous prostate biopsy causing spectral degradation on MRS may result in inaccuracies in the interpretation of the metabolite ratios.
Prostatitis
General Information
Prostatitis is the most common urological disease in men.
Based on the symptomatology and examination of the prostatic expressate, the entity has been classified as acute bacterial prostatitis, chronic bacterial prostatitis, chronic nonbacterial prostatitis/chronic pelvic pain syndrome, and asymptomatic prostatitis.
Nonbacterial causes are responsible for most cases with prostatitis.
Chronic prostatitis usually presents with hematospermia and lower urinary tract symptoms such as dysuria, frequency, urgency, and nocturia.
Imaging
Acute Prostatitis
Ultrasound
The role of ultrasound is limited in patients with acute prostatitis.
TRUS reveals an enlarged prostate with round shape in axial plane, heterogeneous or decreased echogenicity, loss of the echogenicity difference between the TZ and the PZ, and capsular indistinctness. Additionally, a hypoechoic lesion with increased Doppler signal within and around the lesion can be seen.
The main role of TRUS in patients with prostatitis is to exclude prostatic abscess.
A normal TRUS examination usually excludes the possibility of acute prostatitis.
Magnetic Resonance Imaging
On MRI, enlargement of the prostate gland and diffuse decrease of signal intensity or patchy curvilinear regions of alternating low to intermediate signal in the PZ may be detected in acute prostatitis. Extension of the inflammatory reaction into the periprostatic soft tissues and the seminal vesicles may be demonstrable.
Chronic Prostatitis
Ultrasound
The cardinal TRUS findings of chronic prostatitis are listed in Table 2.2. A thin, hypoechoic rim at the outer periphery of the prostate suggests the presence of stromal fibrosis associated with an area uninvolved by inflammation.
Table 2.2
Ultrasound and CDUS findings of chronic prostatitis
Normal prostate size
Heterogeneous or increased echogenicity
Hypoechoic peripheral rim
Focal hypoechoic areas with patchy distribution
Ejaculatory duct calcification
PGZ irregularity
Capsular deformity and/or thickening
Dilatation of periprostatic venous plexus
Increased vascularity on CDUS
Focal atrophy and infarction
Dystrophic prostatic calculi
Dystrophic prostatic calculi may be seen in association with prostatitis.
Focal atrophy and infarction may simulate PC.
Magnetic Resonance Imaging
MRI findings in chronic prostatitis include diffuse decrease of signal intensity or triangular or patchy hypointense areas on T2-weighted images, stringy contrast-enhancing signal alterations without a nodular shape, and well-circumscribed capsular margins without pericapsular signal changes (Fig. 2.16a, b).
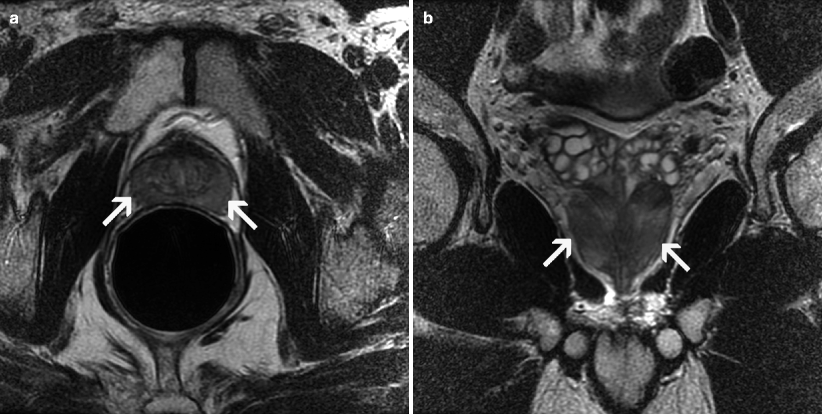
Fig. 2.16
Chronic prostatitis. Axial (a) and coronal (b) T2-weighted MR images demonstrate patchy hypointense areas in the PZ representing chronic prostatitis (arrows)
Prostatic Abscess
Symptoms of prostatic abscess include fever, chills, urgency, perineal pain, dysuria, and hematuria.
Most prostatic abscesses occur as a complication of acute bacterial prostatitis, most often in the fifth and sixth decades of life and most often in diabetic patients. Escherichia coli is the most common causative organism. Abscess may involve any part of the prostate, but the TZ away from the midline is a common site.
Ultrasound
Sonographic findings include focal enlargement of the prostate gland and uni- or multilocular fluid collections with irregular or well-defined walls, containing internal echoes and septae (Fig. 2.17).
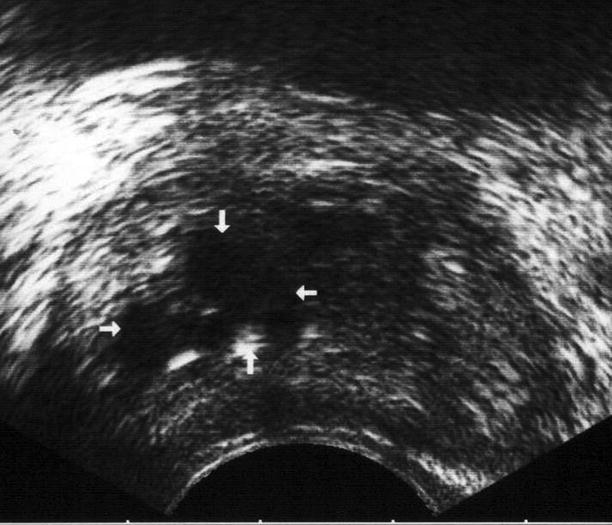
Fig. 2.17
Prostatic abscess. Transverse TRUS image reveals an ill-defined, multiloculated prostatic abscess with heterogeneous internal echotexture (arrows)
A peripheral halo with increased glandular and perilesional vascularity can also be seen. Most prostatic abscesses are detected in the TZ, although any part of the gland may be involved. Seminal vesicles may be affected.
Computed Tomography
CT may be helpful when extension of the abscess beyond the prostate is suspected. CT is also helpful in diagnosing emphysematous prostatitis, a rare entity.
CT examination demonstrates an enlarged prostate with stranding of periprostatic fat. Uni- or multiloculated lesions are seen and may demonstrate wall enhancement, fluid attenuation, and/or internal septations.
Magnetic Resonance Imaging
MRI reveals uni- or multiloculated fluid collections with low signal intensity on T1-weighted and high signal intensity on T2-weighted images as well as an enhancing rim on post-contrast images.
Granulomatous Prostatitis
Granulomatous prostatitis is an unusual benign inflammatory condition of the prostate, which can be mistaken for PC both clinically and sonographically.
Imaging
To date, no US or MRI feature yet described in the literature allows a specific diagnosis of granulomatous prostatitis, and the entity can be differentiated from PC only by histopathological evaluation.
Ultrasound
The term “granulomatous prostatitis” encompasses a group of morphologically distinct forms of chronic prostatitis, some with an identifiable etiology and some in which the pathogenesis is indeterminate.
Tuberculosis is the most common type of infective granulomatous prostatitis. It affects men 20–40 years of age. Primary TB of the prostate is rare, whereas secondary involvement of the gland by TB occurs after the passage of the urine through the prostatic urethra.
TB can also involve the ejaculatory ducts, resulting in stricture formation. Bacillus Calmette-Guerin (BCG) therapy for bladder cancer increases the risk for prostate TB.
The most common TRUS finding of prostate tuberculosis (TB) is irregular hypoechoic areas in the PZ. On TRUS, prostatic enlargement and focal or multifocal hypoechoic lesions may be seen.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree