Chapter 103 Overview: Duodenal atresia and stenosis represent a spectrum ranging from complete to partial obstruction, presenting from prenatal life to late childhood or even adulthood. The incidence of duodenal atresia or stenosis is cited as approximately 1 : 7000 live births and represents nearly half of intestinal atresias.1,2 Similar to atresia of the more distal bowel, duodenal atresia is classified as types I through III. Type I atresias consist of a completely or partially obstructing membrane, type II atresias are connected by a fibrous cord, and type III atresias are separated by a gap. The duodenal diverticulum or “windsock” duodenum is considered a variant of type I atresia.3 Etiology: The lumen of the duodenum is obliterated during the fourth to sixth weeks of gestation because of rapid cell division and normally recanalizes by the twelfth week. The etiology of duodenal atresia and stenosis is believed to be the result of failure of recanalization of the lumen of the duodenum.4,5 Clinical Presentation: Presentation may occur prenatally with polyhydramnios, which occurs in approximately 30% to 50% of cases,3,5 and premature birth is seen in nearly half of patients.5 Approximately one third of cases of congenital duodenal obstruction presenting in the neonatal period are due to duodenal stenosis.5 Postnatally, infants with atresia typically present with vomiting within the first 24 hours of life. Because duodenal atresia/stenosis typically occurs distal to the ampulla of Vater, these patients will present with bilious vomiting. However, obstruction occurs proximal to the ampulla of Vater in as many as 23% of patients.6,7 Such patients will present with nonbilious vomiting, simulating hypertrophic pyloric stenosis. Because duodenal obstruction is proximal, abdominal distension is not a typical finding, although fullness in the epigastric region may be present as a result of the dilated stomach and duodenum. Patients with duodenal stenosis may present later in life, depending on the degree of obstruction; presentation may be relatively nonspecific (such as failure to thrive), or it may present as pancreatitis as a result of reflux into the pancreatic duct, proximal to the site of obstruction.8,9 Presentation may be precipitated by ingestion of a foreign body, which fails to pass and may exacerbate the degree of obstruction, leading to abdominal pain and/or vomiting.10 In patients with a duodenal diaphragm, an intraluminal duodenal diverticulum or “windsock” may be found, and presentation also may be precipitated by ingestion of a foreign body.11 Associated anomalies are common in patients with duodenal atresia/stenosis and may be responsible for presenting signs and symptoms. Annular pancreas and malrotation are seen in approximately one third of cases.5 Trisomy 21 is present in approximately 25% to 40% of infants with duodenal atresia/stenosis; conversely, approximately 4% of infants with Down syndrome have duodenal obstruction.5,12 Hirschsprung disease has been reported in approximately 1% to 3% of patients with duodenal atresia and Down syndrome.13,14 Duodenal atresia may be seen in approximately 5% of patients with esophageal atresia with or without a fistula.15 Heterotaxy with polysplenia has been reported in patients with duodenal diaphragm/intraluminal diverticulum.8,16 Imaging: Abdominal radiographs are the starting point in the evaluation of a child with suspected obstruction. In the neonate with duodenal atresia, the abdominal radiograph demonstrates the classic “double bubble appearance,” representing the dilated stomach and duodenum.17,18 Because obstruction has been present in utero, the obstructed proximal duodenum is typically large, approximately one half to one third the size of the stomach (Fig. 103-1); at times the pylorus is wide open, and the two bubbles are not distinctly separated (see Fig. 103-1, B). This appearance on the abdominal radiograph is diagnostic, and contrast studies to confirm the diagnosis are not needed. Rarely, air can be seen distally in a patient with complete atresia; this occurs when the atresia is flanked by the branches of an anomalous bifid common bile duct, with separate insertions into the duodenum above and below the point of atresia. Air or contrast material may be seen refluxing into the anomalous ducts, which allow the contents of the obstructed proximal duodenum to course into the distal duodenum, bypassing the point of obstruction (Fig. 103-2).7,19 In patients with duodenal stenosis, dilatation of the stomach and proximal duodenum and decrease in distal gas is seen commensurate with the degree of obstruction. If the stenosis is mild or the stomach is decompressed via an enteric tube, the plain abdominal radiographs may be nonrevealing (e-Fig. 103-3). In patients with duodenal atresia associated with esophageal atresia with a fistula, plain films are diagnostic (Fig. 103-4). Figure 103-1 Duodenal atresia. Figure 103-2 Duodenal stenosis with anomalous ducts. Figure 103-4 A 1-day-old infant with esophageal and duodenal atresia. e-Figure 103-3 Duodenal stenosis. An upper gastrointestinal (GI) series may be needed in patients with duodenal stenosis or in cases in which differentiation from malrotation is a concern. Furthermore, malrotation can coexist with duodenal stenosis or atresia (e-Fig. 103-5).20 Therefore if any clinical concern for malrotation exists or if surgery is to be delayed, an upper GI series is indicated. In patients with duodenal atresia, contrast enema has been performed in the past to assess the rotation of the bowel, but this examination is not helpful if it is normal or if the cecum is high-riding.21,22 e-Figure 103-5 Duodenal web. In patients with duodenal stenosis, an upper GI series will confirm the partial obstruction (Fig. 103-6); when a web is present, the membrane may be seen as a thin linear filling defect (see Fig. 103-6, B and C). A duodenal diverticulum is more conspicuous, particularly when underscored by an ingested foreign body (e-Fig. 103-7). In patients with duodenal atresia simulating duodenal stenosis as a result of bypass of the obstruction by anomalous ducts, contrast may be seen within the ducts, as previously discussed (see Fig. 103-2). Figure 103-6 Duodenal stenosis. e-Figure 103-7 Duodenal web with a foreign body. Cross-sectional imaging currently does not have a routine place in evaluation of patients with duodenal atresia or stenosis. However, occasionally a patient with duodenal stenosis proximal to the ampulla of Vater will present with nonbilious vomiting and come to ultrasound with a primary concern of pyloric stenosis. In those cases, ultrasound will reveal an abnormally distended pylorus and dilatation of the duodenal bulb (Fig. 103-8). Treatment: Treatment of duodenal atresia and symptomatic duodenal stenosis is surgical repair. At the time when Ladd reported surgical correction of duodenal obstruction in 1932,23 the reported mortality rate was approximately 40%.24 In 1990, Kimura et al. described the technique of diamond-shaped anastomosis, which has become the standard procedure for open repair, with reported mortality of 5% to 10%, largely due to associated anomalies, particularly those involving cardiac lesions.24,25 More recently, laparoscopic duodenoduodenostomy has been introduced with increasingly good results and reported improvement in return of bowel function, leading to reduced length of hospital stay.24,26 Overview: Annular pancreas refers to encirclement of the descending portion of the duodenum by the pancreatic head. The prevalence of annular pancreas is not known, because asymptomatic cases are not always identified. Autopsy prevalence varies between 1 to 15 : 100,000 adults, whereas endoscopic retrograde cholangiopancreatography (ERCP) studies report 1 to 4 : 1000 among symptomatic patients.27,28 In children the incidence is estimated at approximately 1 to 12 : 15,000 births.29 Annular pancreas may result in extrinsic duodenal obstruction; however, most cases of duodenal obstruction with annular pancreas are most likely the result of an associated intrinsic duodenal abnormality.3 Etiology: The pancreas arises as a small ventral and a larger dorsal bud from the duodenum. Normally the ventral bud rotates and fuses with the dorsal bud. When the ventral bud becomes tethered to the duodenum prior to rotation, or if the ventral bud fails to rotate completely before fusion, the result is an annular pancreas.3,28,30 The pancreatic annulus, the portion surrounding the duodenum, frequently has a separate duct entering the duodenum, opposite the ampulla of Vater. Duodenal contents may reflux through this duct into the annulus. Clinical Presentation: Annular pancreas may be asymptomatic, and adult presentation has been reported (median age of 47 years) with signs and symptoms consistent with neoplasm, jaundice due to obstruction of the common bile duct, or pancreatitis, because duodenal contents may reflux through a separate pancreatic duct into the annulus.28,31 Pediatric patients typically present at a median age of 1 day at diagnosis with findings related to duodenal obstruction, which are either revealed at prenatal sonography or manifested as vomiting or feeding intolerance soon after birth.28 Older pediatric patients can present with pancreatitis and jaundice. Associated anomalies are common in children and include malrotation, esophageal atresia, anal atresia, and cardiac defects, particularly in patients with trisomy 21 and Cornelia de Lange syndrome12,28; annular pancreas also has been reported in patients with heterotaxy.32 Imaging: Plain radiographs show findings of duodenal dilatation in patients who have an obstruction. An upper GI series will show narrowing of the descending portion of the duodenum (Fig. 103-9, A). Ultrasound may show a ring of pancreatic tissue about the descending portion of the duodenum, although the duodenum might be difficult to follow, and the appearance may resemble a mass at the level of the head of the pancreas (Fig. 103-9, B). A more definitive diagnosis can be made with computed tomography (CT; Fig. 103-9, C to E). Magnetic resonance imaging (MRI) also may show the ring of pancreatic tissue, but MR cholangiopancreatography will show more definitive findings, outlining the annular course of the pancreatic ducts (see Fig. 103-9 F and G), analogous to the findings seen with endoscopic retrograde cholangiopancreatography (e-Fig. 103-10). Figure 103-9 Annular pancreas. e-Figure 103-10 Annular pancreas. Overview: The term “malrotation” indicates that the normal rotation of the midgut was not completed, thus presenting a spectrum of abnormalities that affect both the duodenojejunal and the cecal poles of the midgut, singly or in unison. The prevalence of malrotation is difficult to determine, inasmuch as asymptomatic cases may not be recognized; it has been quoted as high as 1 : 500 live births,33 which seems excessive, as that is similar to the incidence of pyloric stenosis (2 to 5:1000). The bowel is thus suspended from a mesentery that is attached to the posterior abdominal wall and that extends from the left upper to the right lower quadrants (Fig. 103-11). The configuration of the duodenal C-loop mirrors the 270° of rotation that it has undergone.21,34–37 The final steps in bowel rotation and fixation involve resorption of the dorsal mesenteries of the ascending and descending colon and elongation of the ascending colon with descent of the cecum, processes that are ongoing until several months of postnatal life.33 The Meckel diverticulum is the demarcation point between the embryonic prearterial and postarterial limbs. Figure 103-11 Normal rotation of midgut. Etiology: The aforementioned background is important in the understanding of the clinical problems that arise with malrotation and malfixation of the bowel. Malrotation arises when the process of normal rotation is arrested, which can occur at any point along the rotation of the duodenojejunal or cecal segments. If it is arrested after the initial 90° of counterclockwise rotation, the duodenojejunal junction and the small bowel will be located on the right side of the abdomen, and the cecum and the remainder of the colon will be located in the left side of the abdomen. Despite the fact that the initial 90° of counterclockwise rotation have occurred in both the duodenojejunal junction and the cecum, this arrangement is nevertheless known as nonrotation, a specific variant of malrotation, characterized by a relatively long mesenteric pedicle.38 Progression of the rotation process beyond the nonrotation stage brings the duodenojejunal junction and the cecum into proximity, and arrest along this portion of the spectrum typically results in a shorter mesenteric pedicle from which the bowel is suspended (Fig. 103-12), believed to increase the risk for subsequent volvulus. Reversed rotation is a rare form of malrotation in which the gut undergoes 90° of clockwise rotation, instead of the normal 270° of counterclockwise rotation. Starting from the initial straight tube, this rotation results in the location of the duodenojejunal junction in the left upper quadrant and of the cecum in the right lower quadrant. However, in these cases, the ceco-colic loop returns into the abdomen first, which results in the transverse colon, not the duodenum, being located between the aorta and the superior mesenteric artery.39,40 Figure 103-12 Malrotation. Several disorders of fixation also commonly occur in patients with malrotation. Ladd bands are the result of abnormal mesenteric attachments that are formed in patients in whom the rotational process is incomplete. These bands, named after Dr. William E. Ladd, typically extend from the edge of the liver to the malrotated cecum, crossing over and causing obstruction and kinking of the duodenum (Fig. 103-13); however, they can rarely occur more distally, in the jejunum or ileum.34 Figure 103-13 Ladd bands. Internal hernias are the result of abnormal or incomplete fixation of the ascending (right paraduodenal or mesocolic) or descending portions of the colon, resulting in a defect through which the bowel can herniate. Herniation into the defect in the ascending colonic mesentery results in a right paraduodenal or mesocolic hernia, whereas herniation into the defect in the descending colonic mesentery results in a left paraduodenal or mesocolic hernia.34,37 In persons with a normally rotated bowel, abnormal or incomplete fixation of the ascending colon, cecum, or sigmoid colon can result in volvulus of these structures, which typically does not occur until late adult life. Clinical Presentation: The presentation of patients with malrotation depends on whether obstruction is present, and if so, whether the obstruction is acute or chronic, as well as on associated malformations. Presentation may occur in utero, and the infant may be born with short bowel as a result of in utero bowel necrosis and resorption, or with the apple-peel type of atresia.41,42 In asymptomatic patients malrotation may be discovered in the course of evaluation for other clinical concerns. Acute Volvulus: In persons with volvulus, the duodenum rotates, typically clockwise, about the axis of the superior mesenteric artery (Fig. 103-14) and is obstructed at its third portion, distal to the ampulla of Vater. Because approximately 60% to 80% of patients with volvulus present in the first month of life,33,34,38,43,44 the typical presentation is that of a previously well neonate who experiences the sudden onset of bilious vomiting. The infant may experience crampy abdominal pain, which could be confused with colic. If the obstruction is significant, the abdomen may become initially scaphoid after distal intestinal contents are evacuated. Vascular compromise may lead to intraluminal bleeding and hematochezia, seen in 10% to 15% of patients with volvulus.37 As ischemia of the midgut supervenes, the abdomen becomes distended and firm, with physical signs of peritonitis, and the patient will present in shock with cardiovascular collapse.34 Chronic Volvulus: Partial or intermittent volvulus tends to present more insidiously, with an average duration of symptoms of 28 months or greater before the correct diagnosis is made.34 The patient usually has a history of abdominal pain, which can be severe but often is vague and intermittent, and often is associated with intermittent vomiting and/or failure to thrive. Impairment of venous and lymphatic flow leads to signs and symptoms of malabsorption.45,46 The correct diagnosis is frequently delayed, with interim diagnoses including central or psychogenic vomiting, milk or other food allergies, and various malabsorption syndromes, such as celiac disease.20,34,47,48 Ladd Bands: Patients with duodenal obstruction as a result of Ladd bands may present acutely, with sudden onset of bilious vomiting, or they may present with more chronic symptoms, with failure to thrive and intermittent abdominal pain.34 Associated Conditions: Multiple malformations and conditions are associated with malrotation and are reported to occur in 30% to 60% of patients with malrotation.34,38 Intrinsic duodenal stenosis and annular pancreas have been discussed previously, and other intestinal atresias also occur.5,38 Other abnormalities include Hirschsprung disease and anorectal malformations, cloacal extrophy, Eagle-Barrett (prune belly) syndrome, megacystis-microcolon-intestinal hypoperistalsis or Berdon syndrome, Cornelia de Lange syndrome, Marfan syndrome, and Meckel syndrome.21,37,38,49 Malrotation also is found in some patients with trisomy 13, 18, and 21, in whom malrotation is cited as being 25 times more common than in the general population.21,50 Malrotation is part of anomalies in which the gut is unable to achieve its normal rotation—gastroschisis, omphalocele, and Bochdalek diaphragmatic hernia—and is also extremely common in patients with heterotaxy.51–53 Imaging: Plain abdominal radiographic findings in patients with malrotation without obstruction or volvulus may show an abnormal distribution of stool, with absence of stool pattern in the right lower quadrant, which has been termed the radiologic “Dance’s sign.”48 In patients with nonrotation, all colonic stool may be seen as confined to the left half of the abdomen. In patients with volvulus, the radiographs may be completely normal at the onset of the bilious vomiting (Fig. 103-15, A). As distal bowel contents are evacuated and greater obstruction supervenes, the radiographs may show distension of the stomach, with relatively minor or subtle distension of the duodenum (Fig. 103-15, B and C). It is important to note that in malrotation with volvulus, the duodenum is not typically markedly distended, as is the case with duodenal stenosis or atresia; obstruction with Ladd bands is more likely to lead to an appearance more resembling that of the double bubble. The lack of marked bowel distension in an infant with volvulus may lead to a false sense of security or to the suspicion of a more common condition such as pyloric stenosis, if the significance of the bile-stained vomitus is not appreciated. Figure 103-15 Acute midgut volvulus. Ominous plain radiographic findings of ischemia in patients with volvulus include abdominal distension, separation of bowel loops, tubular appearance of bowel loops, fold thickening, and thumbprinting. Diffuse fluid and gaseous distention of the bowel is a finding that suggests gangrenous bowel and a poor prognosis (Fig. 103-15, D). Upper GI Series: An upper GI series is at present considered the standard examination to evaluate for malrotation and for its complications, particularly volvulus. Documentation of normally rotated bowel can be challenging, and technique is very important. In pediatric patients, documentation of the normally rotated C-loop needs to be made on passage of the first bolus of contrast from the stomach; missing this opportunity can result in confusing and misleading findings. The course of the duodenum must be documented in both frontal and lateral projections, because the lateral view is essential to document the retroperitoneal position of both ascending and descending portions.37,54 The duodenojejunal junction, recognized by an acute flexure as the duodenum returns to the peritoneal cavity, should be located to the left of the left pedicle at the same craniocaudal level as the duodenal bulb. The lateral view should show ascending and descending portions entirely superimposed anterior to the spine; anterior deviation of the descending limb is abnormal. The technique is discussed in Chapter 85. When malrotation with volvulus is suspected, we typically will use a low-osmolality water-soluble agent and position an enteric tube distally within the stomach to improve control over the first bolus. The fluoro-grab function of most current fluoroscopes allows movielike documentation of the progress of the first bolus through the duodenum without additional radiation dose, and it can be very helpful in confirming normal rotation, as well as in sorting out difficult cases and normal duodenal variants. The radiologist should be familiar with normal variants such as duodenum inversum, in which the duodenum shows a parallel ascending and descending course to the right of the spine, before crossing to the left and emerging into the peritoneal cavity at a normally positioned ligament of Treitz (Fig. 103-16, Video 103-1).55 Conversely, it is imperative that an abnormal course of the duodenum in patients without volvulus be recognized, including abnormal location of the duodenojejunal junction on the frontal image (see Fig. 103-16, C) and an abnormally anterior course of all or part of the duodenum on the lateral view. Abnormal location of the duodenojejunal junction—“excessive redundancy of the duodenum to the right of the spine”—may be indicative of malrotation55 and other findings, such as position of the duodenum on the lateral view, position of the cecum, and cross-sectional imaging may need to be considered. Figure 103-16 Upper gastrointestinal imaging (UGI) of the duodenum. In symptomatic patients with malrotation, the upper GI examination may reveal the volvulus itself, presenting a spiral twisting of the duodenum (classically described as a “corkscrew”) as it wraps around the axis of the superior mesenteric artery (see Fig. 103-16, D). When complete obstruction is present, the contrast column may terminate in a beaklike configuration as the contrast is propelled into the entrance of the spiral but cannot proceed further (see Fig. 103-16, E). In patients with obstruction resulting from Ladd bands, the duodenum may demonstrate a kinked configuration (see Fig. 103-16, F) that at times resembles a Z-shape instead of the normal C-loop. The Z-shape is the result of tacking down and kinking of the duodenum by the abnormal attempts at fixation and at times may be very difficult to differentiate from volvulus. Contrast Enema: Barium enema had been advocated by surgeons and radiologists in the investigation of patients suspected of malrotation and volvulus, in the belief that cecal position would define the presence or absence of malrotation while avoiding introduction of barium contrast proximal to the obstructed duodenum.20,56 However, with the understanding that up to 30% of patients with malrotation and its complications could have a normally rotated cecum, and with the realization that a high-riding cecum in a neonate may in fact be normally rotated,20,57,58 the contrast enema is no longer considered the main fluoroscopic diagnostic tool in the investigation of these patients. Follow-through small bowel to evaluate the cecum when the upper GI series shows confusing findings has been advocated and may be helpful in some cases, but the previous problems are not obviated. The cecum itself may be very difficult to define if the appendix does not fill, and a colonic segment in the right lower quadrant may be mistaken for the cecum. However, a clearly abnormal cecal position, especially in children with an equivocal location of the duodenal-jejunal flexure, is diagnostic of malrotation. Overview: A clearly abnormal course of the duodenum renders the diagnosis of malrotation straightforward (see Fig. 103-16, C). Unfortunately, subtle cases exist that lead to false-negative interpretations, and as previously discussed, normal variants may lead to false-positive results. In a retrospective review of 163 patients with surgically proven malrotation, upper GI examination had a sensitivity of 96%, with seven false-negative examinations.59 On the other hand, a 15% false-positive diagnosis by upper GI examination, as demonstrated on subsequent surgical findings, also has been reported.60 The frontal course of the duodenum may be misleading if the duodenojejunal flexure occurs over rather than to the left of the pedicle of the spine, if it occurs below the plane of the duodenal bulb, or if abnormal redundancy of the course of the duodenum occurs. The duodenojejunal junction also can be displaced by postoperative changes after liver transplantation,61 peritoneal masses, or abnormal, dilated bowel loops.62 Limitations of the lateral view include the fact that the aorta and the superior mesenteric artery are not visualized, and therefore true retroperitoneal position of the duodenum cannot be ascertained unequivocally, despite the parallel course of its ascending and descending portions anterior to the spine.22 Interpretative Errors: False-positive interpretations usually result from failure to recognize normal variants or from displacement of a normal ligament of Treitz. Position of the proximal small bowel in the right upper quadrant, as an isolated finding, is not indicative of malrotation.55,59 We know that the neonatal duodenum is mobile.58 Therefore in patients in whom there is internal traction upon the duodenum, such as infants with dilatation of bowel, the duodenojejunal junction may appear abnormally located on the upper GI examination.62 Normal variants of duodenal course, such as duodenum inversum (see Fig. 103-16, A and B, and Video 103-1) or redundant duodenum, also may lead to false-positive interpretations, although marked redundancy of the duodenum also can be a sign of malrotation.55 Analysis of the true lateral view, or correlation with cross-sectional information, may be needed in some of these cases. A high position of the cecum or a mobile cecum, particularly in a neonate, also are typically normal findings but make evaluation for malrotation particularly challenging in individual patients. Potential false-negative outcomes may result from suboptimal technique, such as failing to delineate the course of the duodenum on the first bolus of contrast from the stomach or from misinterpretation of the subtle finding of malrotation, such as kinking of the duodenum along its course or location of the duodenojejunal junction abnormally low or to the right of the left pedicle of the spine.21,37,54,58,59 A proposed scoring system whereby the presence of three of nine potential findings would indicate malrotation, whereas one of nine was considered normal and two of nine was considered indeterminate, has not met with universal success.55,58
Congenital and Neonatal Abnormalities
Duodenum
Intrinsic Lesions: Duodenal Atresia and Stenosis
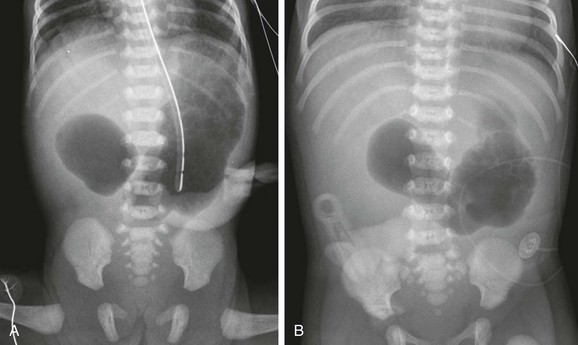
A, A 1-day-old premature infant born at 32 weeks’ gestation with prenatally diagnosed duodenal atresia. The radiograph shows the classic double bubble, with a distended stomach and duodenum and no distal gas. B, A full-term infant with a prenatal diagnosis of duodenal atresia. The radiograph shows a gaping pylorus through which there is wide communication between the dilated stomach and the dilated duodenum, with no distal gas. At surgery, an associated malrotation without volvulus was discovered, and a Ladd procedure was performed, in addition to a duodenoduodenostomy.
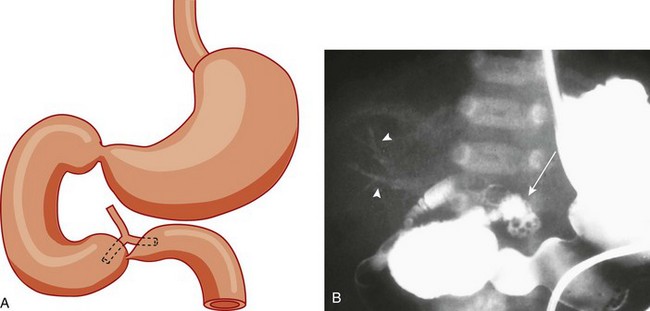
A, A schematic rendering of duodenal obstruction bypass by dual anomalous ducts. B, An upper gastrointestinal series in a neonate with duodenal atresia shows contrast material within the ductal system (arrowheads) and within the bowel lumen beyond the site of obstruction.
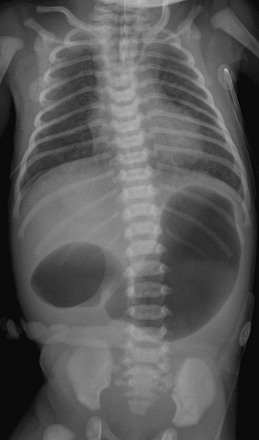
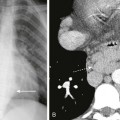
Note the orogastric tube in the proximal esophageal pouch; abdominal double bubble indicates a distal tracheoesophageal fistula and duodenal atresia.
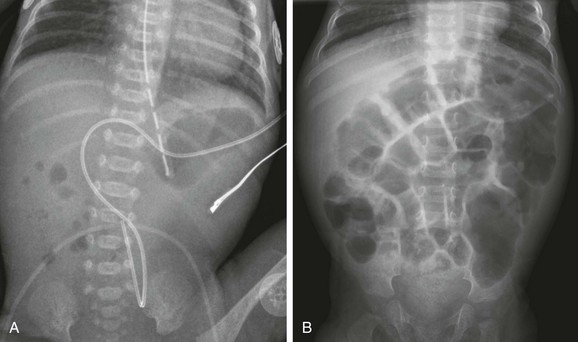
A, A 1-day-old premature twin infant with bilious aspirates. The radiograph demonstrates paucity of distal bowel gas, which could be ascribed to decompression of the stomach by the orogastric tube. Duodenal stenosis was shown on a subsequent upper gastrointestinal examination and confirmed at surgery. B, An 8-month-old with Down syndrome who was admitted for investigation of failure to thrive. Although the radiograph does not suggest duodenal obstruction, duodenal stenosis with a membrane was demonstrated upon upper gastrointestinal examination and confirmed at subsequent surgery.
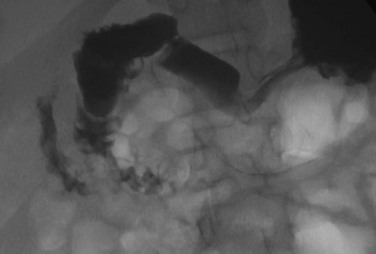
An upper gastrointestinal examination in an adolescent with a history of intermittent abdominal pain shows a duodenal web and malrotation.
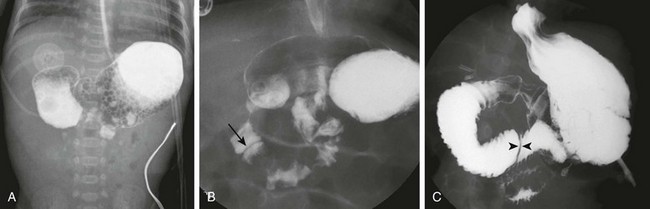
A, The same infant as shown in e-Figure 103-3, A. A 30-minute radiograph after upper gastrointestinal (GI) examination shows the duodenal obstruction. B, The same infant as shown in e-Figure 103-3, B. An image from an upper GI examination shows a duodenal web (arrow). C, Image from an upper GI examination in a 9-month-old girl demonstrates a thin duodenal web (arrowheads).
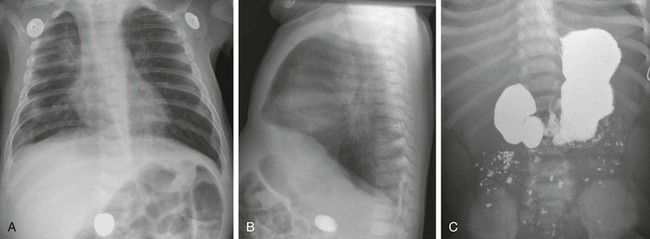
Anteroposterior (A) and lateral (B) chest radiographs in a 6-month-old demonstrate a foreign body (a rock ingested several weeks previously) in the right mid abdomen. C, A subsequent upper gastrointestinal examination demonstrates a dilated duodenum proximal to a confirmed web (the rock is obscured by the barium).
Extrinsic Lesions: Annular Pancreas, Malrotation, Preduodenal Portal Vein, and Duplication Cysts
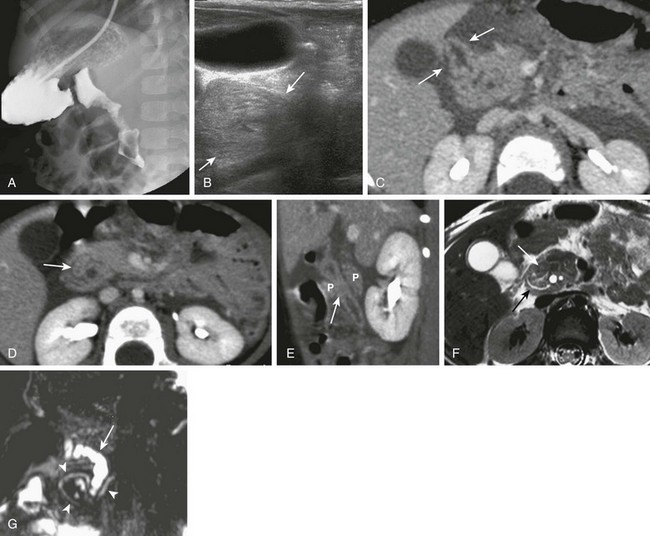
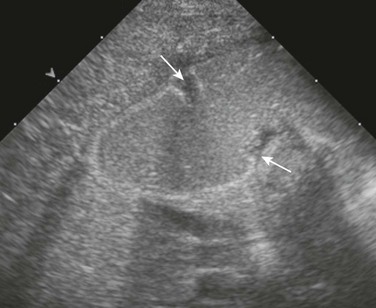
A, An upper gastrointestinal (UGI) examination in a 2-year-old girl with annular pancreas demonstrates a circumferential impression upon the descending duodenum. The patient had presented with pancreatitis; the UGI examination was performed after her symptoms had subsided, to assess for an underlying element of duodenal obstruction. B, Ultrasound at the time of initial presentation shows a thickened head of the pancreas (arrows); the central duodenum was not identified. C-E, Abdominal computed tomography images. C, The duodenum (arrows) is seen entering the head of the pancreas. D, The duodenum (arrow) is again seen within the head of the pancreas, just lateral to the common bile duct. E, Sagittal reformat shows the duodenum (arrow) with pancreatic tissue (P) anterior and posterior to its descending portion. Free fluid is secondary to pancreatitis. F, Abdominal T2-weighted magnetic resonance (MR) shows the duodenum (white arrow) within the head of the pancreas, with circumferential pancreatic tissue, outlined by a portion of the encircling pancreatic duct dorsally (black arrow). G, MR cholangiopancreatography delineates the pancreatic duct (arrowheads) and its circumferential course at the head of the pancreas. The arrow points to the dilated common bile duct. (Courtesy Melissa A. Hilmes, Nashville, TN.)
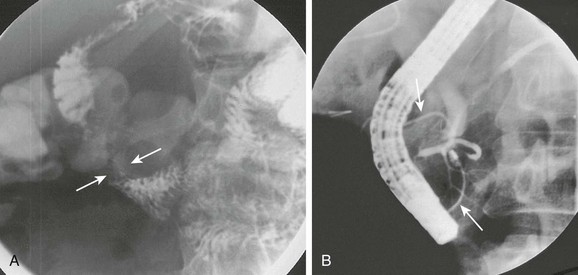
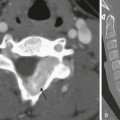
A, Oblique view of the duodenal C-loop during an upper gastrointestinal series shows extrinsic narrowing (arrows). B, Similar view as in A. This endoscopic retrograde cholangiopancreatography image confirms a circumferential pancreatic duct (arrows) and annular pancreas. (Courtesy Dr. George Taylor, Boston, MA.)
Malrotation
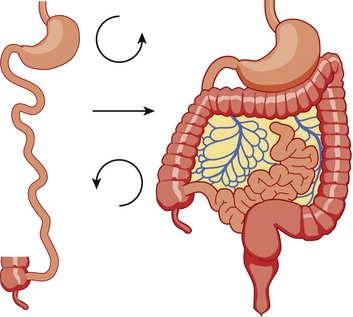
The midgut starts as a straight tube; 270 degrees of counterclockwise rotation of the duodenojejunal junction places it in the left upper quadrant, whereas 270 degrees of counterclockwise rotation of the cecum places it in the right lower quadrant. Normal fixation of the bowel after rotation is completed results in the midgut being suspended from a broad-based mesentery, with retroperitoneal attachment at both ends.
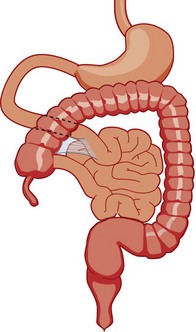
As the gut rotation is arrested between the initial 90 degrees (“nonrotation”) and the normal 270 degrees, the duodenojejunal junction and the cecum approximate, resulting in suspension of the midgut from a narrow mesenteric pedicle without normal attachments. This arrangement is at high risk for midgut volvulus.
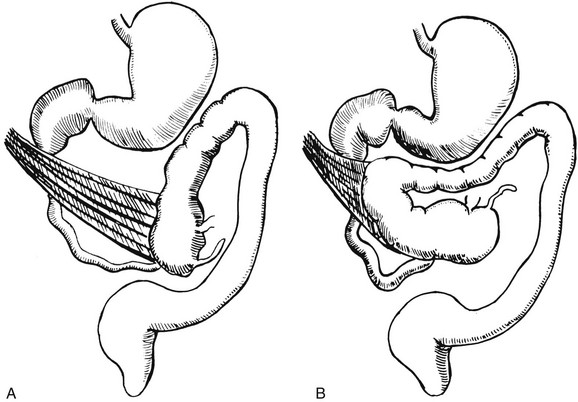
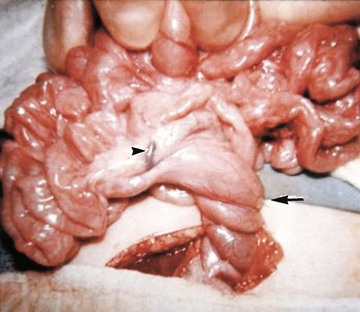
Illustration of two instances in the spectrum of malrotation, demonstrating dense peritoneal bands (Ladd bands) extending from the cecum to the right upper quadrant, crossing over and obstructing the duodenum. These bands must be divided after reduction of the volvulus to relieve the obstruction.
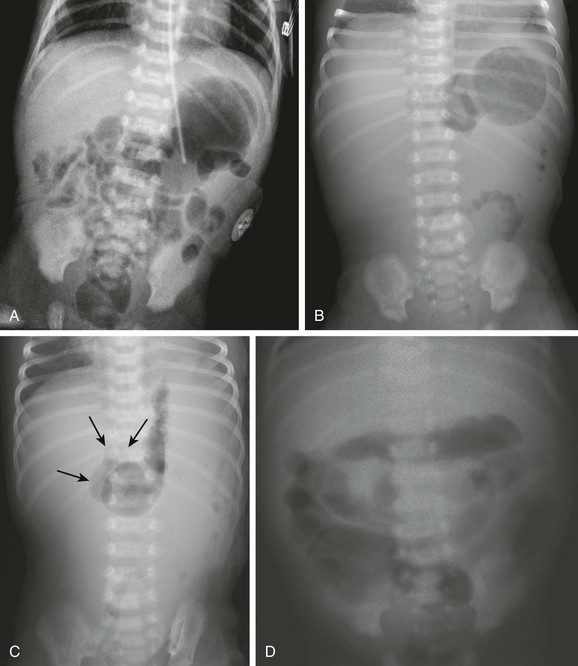
Abdominal radiographic findings. A, An abdominal radiograph in 2-day-old infant with bilious vomiting and midgut volvulus. The bowel gas pattern is easily interpreted as within normal limits. B and C, Supine (B) and left decubitus (C) radiographs in a 7-day-old infant with bilious vomiting and midgut volvulus. The bowel gas pattern is abnormal, with very little gas distal to the stomach, and a scaphoid abdomen. No distension of the duodenum is appreciated, and the stomach is normal in size, but there is discrepancy between the size of the stomach and the marked lack of gas distally. On the decubitus radiograph, distension of both the stomach and the duodenum (arrows) is appreciated, with remarkable paucity of distal gas. D, A 2-week-old infant with bilious vomiting for several days. The radiograph shows a severe ileus pattern with abdominal distension and fluid and gas distending loops of bowel throughout the abdomen. The bowel was necrotic at subsequent surgery, and the infant died.
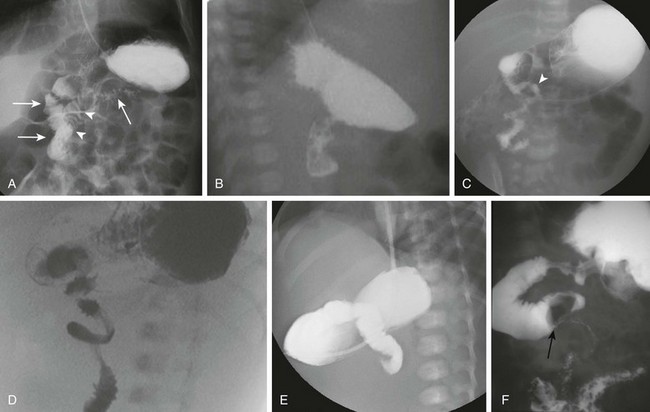
A and B, Normal variant of duodenum inversum. A frontal image (A) of an infant with duodenum inversum. Note that after the vertical course of the duodenum on the right (left, parallel arrows), it ascends (arrowheads) before crossing the midline to a normally positioned duodenojejunal junction at the ligament of Treitz (right arrow). A lateral view (B) in a patient with duodenum inversum shows normal posterior position of the duodenal course. C, Malrotation in an infant without volvulus. A frontal image demonstrates that the presumed duodenojejunal junction (arrowhead) is low and to the right of the midline. D, A fluoro-grab image obtained during UGI in the same 7-day-old patient illustrated in Figure 103-15, B and C. Note the classic corkscrew configuration of the duodenum as it wraps around the twisted mesenteric pedicle. E, A UGI on another 7-day-old infant with a 1-day history of bilious vomiting and dehydration. The duodenum was completely obstructed, terminating in a configuration resembling a beak. F, Ladd bands in a 2-month-old girl with malrotation. A left posterior oblique spot UGI from a series demonstrates a dilated proximal duodenum as a result of crossing bands at the level of the third portion of the duodenum. The presumed duodenojejunal junction is abnormal. Note the kinking of the duodenum (arrow).