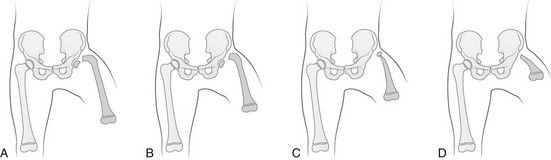
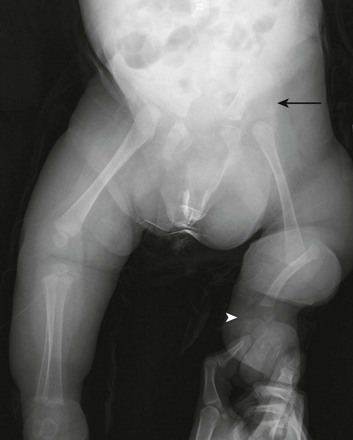
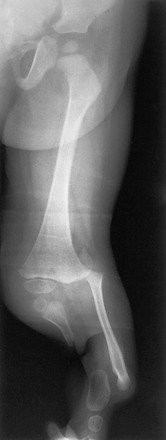
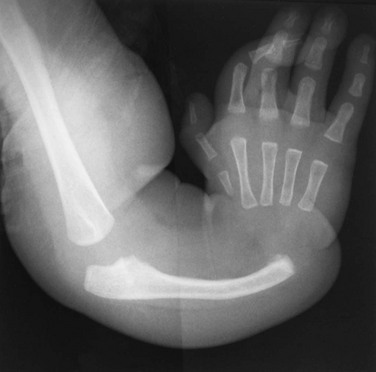
Chapter 132 Regardless of origin, abnormal limb development tends to fall into patterns that can be recognized clinically and radiographically (Fig. 132-1). Most classification systems of congenital malformations are based on osseous structures, but it is well recognized that anomalous conditions of surrounding soft tissues are certain to be present. Although one deficiency is usually dominant, dysplasia often involves the entire limb. Figure 132-1 Skeletal deformities of the limbs. Congenital deficiencies are more common than acquired amputations in children. Frantz and O’Rahilly developed a system of classification that is still commonly used to evaluate congenital anomalies of the extremities.1 Each malformation is defined by the part that is deficient. For example, the fibula is deficient in fibular hemimelia. The abnormality is considered terminal if the deformity extends to the distal aspect of the extremity; it is intercalary if the limb distal to the deformity is normal. For example, in fibular hemimelia, if the foot is abnormal, the deficiency is terminal, and if the foot is normal, it is intercalary. Likewise, a defect may be classified as longitudinal (paraxial) or transverse. The malformation is paraxial if only the fibular side is affected. If both the tibia and the fibula are affected, the deformity is considered transverse. The cause of reduction malformations is known in only a small number of cases. A few abnormalities are heritable, but most are sporadic. Environmental factors are implicated infrequently. Medications such as thalidomide induced malformations that were reduction deformities, ranging from severe amelias to mild muscular hypoplasia.2 Most malformations were on the radial or tibial side, and the upper limbs were affected more often than were the lower extremities. Infrequently, excess deformities such as polydactyly were observed. Proximal Femoral Focal Deficiency Etiology: Proximal femoral focal deficiency (PFFD) refers to abnormalities that range from mild shortening and hypoplasia of the femur to severe deficiency of the bone and dysplasia of the acetabulum. The defect is thought to be a result of altered proliferation and maturation of the chondrocytes of the proximal femoral physis in utero, which in turn results in underdevelopment of the ipsilateral acetabulum. A child with PFFD usually presents in infancy with a short extremity and an unstable hip.3 A well-formed acetabulum implies the presence of a femoral head, which might be cartilaginous and therefore is not apparent radiographically early in life. Most cases are sporadic; however, the combination of bilateral femoral deficiencies and abnormal facies, known as the femoral hypoplasia–unusual facies syndrome, is thought to be an autosomal-dominant disorder. PFFD should be distinguished from developmental dysplasia of the hip, infantile coxa vara, and congenitally short femur. In the setting of developmental dysplasia of the hip, proximal femoral neck and head hypoplasia may be present. The subtrochanteric region of the femur is normal and distal congenital anomalies are absent, and the deformities at the level of the acetabulum are more severe than the deformities at the level of the femoral head and neck. Persons with infantile coxa vara have a normal subtrochanteric region and distal bones, and the femur is normal length, accounting for bowing. Unlike PFFD, infantile coxa vara is discovered after weight bearing occurs.4 A congenitally short femur may be unilateral or bilateral and often is associated with reduction defects elsewhere in the same limb. Most cases are sporadic, but several external factors have been implicated in more complex deformities. Known causes include drugs (such as thalidomide), trauma, irradiation, infection, and focal ischemia.5 Unlike patients with PFFD, patients with a congenitally short femur have a stable hip. Imaging: The most commonly used classification for PFFD was proposed by Aitken (Fig. 132-2).6 The least severe type is class A, which refers to an adequate or only mildly dysplastic proximal femur and acetabulum. The femoral head is present but is separated from the shortened distal femoral segment. With age, a fibrous connection between the head and the distal femoral segment ossifies—usually, not completely. A subtrochanteric varus deformity invariably is present. Class C deformities have a residual acetabulum present (Fig. 132-3). In the most severe form, class D, most of the femur and the ipsilateral acetabulum are absent. Figure 132-3 Aiken type 3 proximal femoral focal deficiency of the left hip in a 4-week-old boy. PFFD is associated with other ipsilateral deformities, including fibular hemimelia (in more than 50% of affected children), shortening of the tibia, equinovalgus deformity of the foot (more often than equinovarus deformity), and deficiency of the lateral rays of the foot. PFFD is bilateral in 15% of affected children.7 Sonography can be used to delineate radiographically inapparent structures at an early age. If a cartilaginous femoral head and its connection to the femoral shaft can be shown, the stability of the hip joint is likely greater than is implied from radiographs. Similar to sonography, magnetic resonance imaging (MRI) is useful for defining the anatomy of the hip joint and associated deformities of the limb,8 allowing for appropriate early classification of deformity. Although the acetabulum might appear developed, even the least severe forms of PFFD are associated with acetabular deficiency. In contrast to the predominantly anterior deficiency of developmental acetabular dysplasia, the acetabulum in PFFD usually has an insufficient posterior wall. Most of the muscles about the affected hip are hypoplastic when compared with the contralateral normal side, with the exception of the sartorius muscle, which often is hypertrophied. This situation results in the characteristic flexion deformity of the hip and knee. MRI also defines the anatomy of soft tissues that can guide surgical exposure and preparation of the limb stump for fitting of a prosthesis. Treatment and Follow-up: Therapy for children with PFFD is based on the severity of the deficiency and projection of growth and final limb length discrepancy at maturity. Objectives for treatment are to maximize the length of the extremity, to promote stability of the hip, knee, and ankle, and to optimize anatomic alignment. Mild deformities may not require surgery. Stabilization of the upper femoral defect is controversial and usually is indicated if a deformity is progressive. Children with severe deformities benefit from amputation of the foot and fusion of the knee. Rotationplasty of the tibia, in which the foot is rotated 180 degrees (so that the toes face posteriorly and the ankle now serves as a knee joint), allows the ankle and foot to control a distal prosthesis.9 Intact sensory feedback from the foot provides proprioceptive control of the knee. Thus it is imperative to assess the morphology of the proximal femur and acetabulum, the cartilaginous epiphyses of the knee, and the supporting soft tissue structures. For example, instability of the knee can result from absence of the anterior cruciate ligament. In the rare case of bilateral PFFD, amputation is contraindicated and therapy is based on extension prostheses that enhance the child’s height. Etiology: Fibular hemimelia is the most common hemimelia and the most common congenital anomaly of the fibula. It also is referred to as congenital short tibia with absent fibula syndrome. Unilateral absence is more frequent than bilateral deformity. A band of strong connective tissue may replace all or most of the fibula.10 Fibular hemimelia is associated with a short, bowed tibia, absence of lateral rays of the foot, tarsal abnormalities (particularly coalitions), and femoral shortening or deficiency in 15% of patients. Caskey and Lester11 reported a clubfoot deformity in 16% of patients with fibular hemimelia. Other associations include small, subluxed, or dislocated patellae, hypoplastic femoral condyles, and ligamentous deficiencies of the knee. Imaging: Fibular hemimelia ranges from mild deficiency of the proximal end of the bone to complete absence accompanied by multiple malformations of neighboring structures (Fig. 132-4). MRI can document associated abnormalities, particularly if surgery is contemplated, including associated ligamentous deficiencies of the knee. Treatment and Follow-up: The talipes equinovalgus deformity of the foot and severe shortening of the limb associated with fibular hemimelia result in poor function of the limb. Children with extensive abnormalities involving the fibula, ipsilateral tibia, femur, and foot benefit the most from early amputation of the foot and often the more proximal structures.12 If the deformity is less severe, lengthening of the affected side, realignment of the tibiotalar articulation, and epiphysiodesis of the contralateral limb often are undertaken. Attempts at lengthening a severely dysplastic limb often are unsatisfactory. Etiology: Most cases of tibial hemimelia are sporadic, although reported forms (particularly bilateral deformities) display an autosomal-dominant inheritance pattern. Jones et al.13 classified the spectrum of tibial dysplasia into four groups. In type I, which is the most severe form, the tibia is not recognized radiographically at birth. Lack of ossification of the distal femoral epiphysis implies that no proximal tibia is present. The least severe form, type IV, includes congenital tibial diastasis. In children with this form, the tibia is short and diverges from the fibula at the ankle. The talus is displaced proximally. No normal distal tibial articulation surface is present. Imaging: Radiography of tibial hemimelia (Fig. 132-5) ranges from absence of a portion of the distal tibia to complete absence with multiple malformations of neighboring structures. MRI can document associated abnormalities, including associated muscular and ligamentous deficiencies, which is important if surgery is contemplated. MRI may identify a cartilaginous tibial remnant that may not be identified readily on radiography. Treatment and Follow-up: Treatment of tibial hemimelia varies with the severity of the deformity. A knee disarticulation is recommended for complete tibial absence. Brown pioneered centralization of the fibula with a Syme amputation of the foot in children with complete tibial hemimelia.14 This approach produces adequate results in the rare cases in which the quadriceps muscles are well developed. When the quadriceps mechanism is abnormal, the patella often is absent. MRI can be used to assess deficiencies preoperatively, allowing the clinician to discern who will function better with primary knee disarticulation (most patients) rather than knee reconstruction. Etiology: Conditions associated with radial deficiency are listed in Box 132-1.15 Usually the entire limb is involved to some degree, which often results in joint dysfunction of the shoulder, elbow, wrist, carpus, and small joints of the hand. Associated muscular deficiencies also are proportionate to the degree of skeletal abnormality. Neurovascular abnormalities include an absent radial artery and superficial radial nerve. Whether radial deficiency is isolated or is associated with a syndrome, the primary malformation is likely the result of a vascular abnormality in the embryo that occurred before differentiation of mesenchyme into muscle and bone.16 Most cases are sporadic, but some autosomal-dominant and autosomal-recessive inheritance patterns are reported. Other cases are likely the result of various environmental insults from viruses, chemicals, radiation, and drugs during limb bud development. Imaging: Congenital radial deficiency ranges from hypoplasia of the thumb to various degrees of radial hypoplasia. Complete absence is the most common longitudinal deficiency. This deformity usually is accompanied by radial and volar deviation of the hand, which results in part from unopposed pull by the flexor carpi radialis and brachioradialis muscles. In most cases of radial aplasia, the forearm is bowed to the radial side and the distal ulna is prominent (Fig. 132-6). The forearm is short (usually approximately two thirds the length of the normal contralateral side) and remains proportionately so throughout growth. Often, only the capitate, hamate, and triquetral bones and the metacarpals and phalanges of the four ulnar rays are present and normal. The trapezium, scaphoid, and first ray often are deformed or absent. If a remnant of the radius is present proximally, it usually is fused to the ulna, which is curved, shortened, and thickened. Bilateral deficiency occurs in approximately 50% of affected children.15 The degree of deformity of the hand is related to the severity of deficiency of the forearm. Treatment and Follow-up: Treatment of a longitudinal radial deficiency begins with serial casting and splinting intended to improve radial deviation by stretching the soft tissues. This treatment often is followed by surgical centralization of the hand over the ulna to improve function and appearance.17 Thumb reconstruction and pollicization of the index finger often are indicated. Etiology: Deficiency that occurs along the ulnar or postaxial border of the upper extremity is known as ulnar deficiency or ulnar clubhand. It is a relatively uncommon disorder that occurs much less frequently than radial deficiency (radial clubhand). Approximately 48% of cases have anomalies of the contralateral limb.18 Most cases are sporadic, but associations with many other syndromes have been reported, most commonly Brachmann-de Lange syndrome. Imaging: In persons with ulnar deficiency, coexisting abnormalities almost always are present in the carpal, metacarpal, and phalangeal rays (e-Fig. 132-7). The pisiform is always absent, and the hamate frequently is not detected. Syndactyly, carpal fusion, and radiohumeral fusion are frequent. The forearm is shortened and bowed, with a concavity to the ulnar side. The hand is deviated in an ulnar direction, and elbow abnormalities are common. Hypoplasia of the shoulder girdle and the upper arm can coexist with ulnar deficiencies. Anomalies of the contralateral upper limb and the lower limbs (such as PFFD) have been reported. e-Figure 132-7 Ulnar deficiency. Treatment and Follow-up: Treatment for ulnar deficiency includes early correction of ulnar deviation of the hand, which is achieved with serial casting. Surgical treatment is reserved for cases with significant limitation of function.19 Forearm instability and associated hand deformities must be addressed. Etiology: Constriction bands are thought to result from intrauterine rupture of the amnion and subsequent mechanical constriction of fetal limbs.20 Various body parts become entangled in the amnion as it separates from the chorion. In some instances, adjacent structures are pulled together by the bands and ultimately become fused, producing a soft tissue syndactyly. Bony syndactyly is very rare. The earlier the amnion ruptures, the more severe are the malformations. Limb abnormalities range from slight soft tissue grooves to transverse intrauterine amputations. Acrosyndactyly, craniofacial and visceral anomalies, and fetal death are part of the spectrum of congenital constriction band syndrome. Sporadic congenital constriction band syndrome is estimated to occur at an incidence of 1 : 1200 to 1 : 15,000 births. An alternative explanation is the intrinsic theory, which suggests that an inherent defect leads to bands during limb embryogenesis.20
Congenital Anomalies of Bone
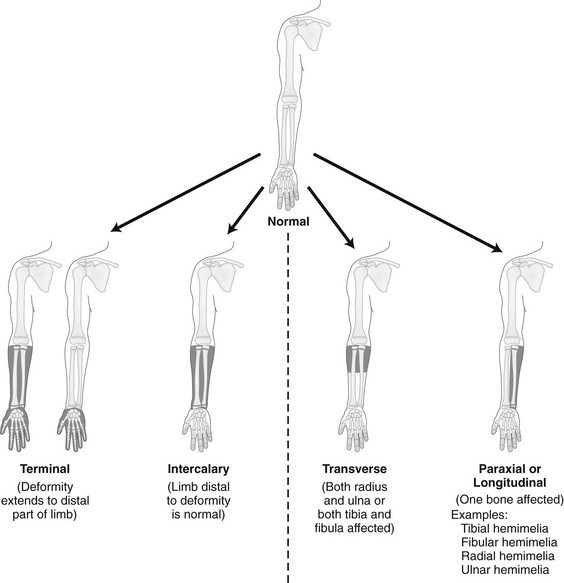
The shaded, blackened areas indicate deficient parts.
Extremity Deficiencies
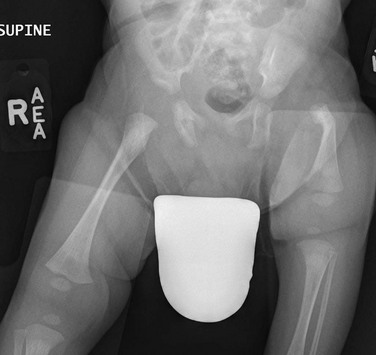
The femur is substantially short and the left acetabulum is severely dysplastic. Mild developmental dysplasia of the right acetabulum is present.
Fibular Hemimelia
Tibial Hemimelia
Radial Deficiency (Radial Dysplasia, Radial Clubhand)
Ulnar Deficiency (Ulnar Dysplasia, Ulnar Clubhand)
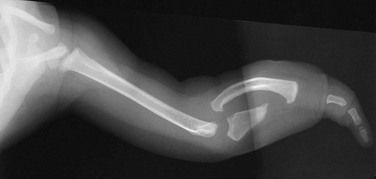
Partial formation of the proximal ulna is present, with aplasia of the distal portion. The radius is bowed, and only a single digit of the hand is present. The humerus is normal.
Generalized Anomalies