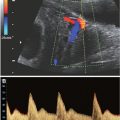
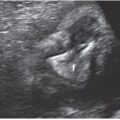
FIGURE 23.1: Sagittal-oblique ultrasound view demonstrating echogenic bowel at 26 gestational weeks in a fetus with known congenital CMV.
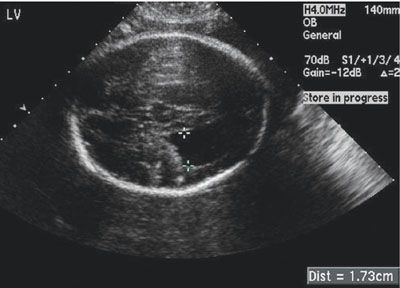
FIGURE 23.2: Axial ultrasound view showing ventriculomegaly in a patient with known congenital CMV at 27 weeks’ gestation.
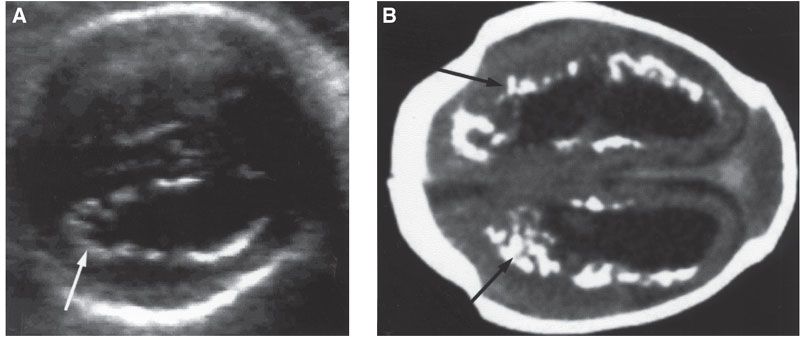
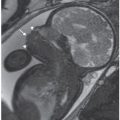
FIGURE 23.3: Cytomegalovirus. A: Transverse view of fetal brain shows ventricular dilatation and periventricular echogenic nodules (arrow). B: Computed tomography scan (oriented to correspond with ultrasound image) after birth confirms hydrocephalus and marked periventricular calcifications (arrows). (Courtesy of Luis Izquierdo, MD, Miami, FL.)
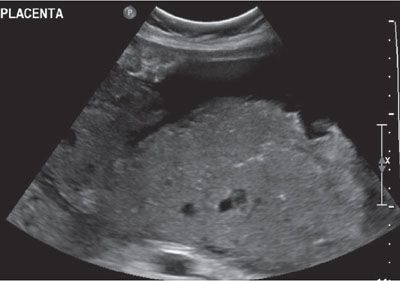
FIGURE 23.4: Thickened placenta in a pregnancy diagnosed with congenital CMV at 30 weeks’ gestation.
Ventriculomegaly is frequently diagnosed during fetal life and has multiple etiologies, including CMV infection. Around 5% of all cases of ventriculomegaly are caused by fetal infection. De Vries et al.14 found periventricular calcifications associated with mild-to-moderate ventriculomegaly in 10 out of 11 children with symptomatic congenital CMV infection. Malinger et al. demonstrated similar findings in five of eight affected fetuses with congenital CMV. In addition, fetuses with ventriculomegaly were identified at a more advanced gestational age (28.6 weeks) and had other associated sonographic findings than those without ventriculomegaly.15
Microcephaly is associated with a poor prognosis in cases of congenital CMV, usually due to mental retardation. Noyola et al.16 reported that microcephaly is the most specific predictor for mental retardation (100%; 95% CI, 84.5–100) and major motor disability (92.3%; 95% CI, 74.8–90) in children with symptomatic congenital CMV infection. Of note, microcephaly may not be apparent during the second-trimester ultrasound examination and generally is not an isolated finding. More common associated findings include intracranial calcifications, increased echogenicity of the lining of the ventricles, and ventriculomegaly.
Ventriculitis, which manifests as an increased echogenicity surrounding the lateral ventricles, is an ultrasound finding that may be present in CMV infection, although it also may be a normal variant. As with ventriculomegaly, it will rarely be an isolated ultrasound finding. Periventricular pseudocysts and intraventricular synechiae (IVS) have also been described in association with CMV infection.
Brain calcifications are considered a strong and common ultrasound marker of intrauterine infections. It is not an exclusive ultrasound finding in CMV infection and has been described in fetuses with other infections such as congenital toxoplasmosis, rubella, herpes simplex, and varicella. The calcifications do not usually have an acoustic shadowing, and although they may be found in any portion of the brain, they have particular predilection for the periventricular zone.
Congenital CMV infection during the late first or early second trimester may interfere with neuronal proliferation and migration, and result in abnormal cortical development, including lissencephaly and schizencephaly. Microcephaly, ventriculomegaly, callosal dysgenesis, and abnormally developed sulci and gyration are the most common initial ultrasound findings in fetuses with congenital CMV infection and malformations of cortical development. A detailed ultrasound, including dedicated neurosonography, and perinatal MRI are indicated when the above-mentioned intracranial ultrasound findings are detected during the initial routine screening examination.
Hyperechogenic bowel and ventriculomegaly are the most common abnormal ultrasound findings in CMV infection, although they may also be seen in uninfected fetuses. Guerra et al.17 reported the following sonographic abnormalities in 23 cases of CMV-infected fetuses/infants: hyperechogenic bowel (n = 7), cerebral ventriculomegaly (n = 7), IUGR (n = 3), hydronephrosis (n = 1), hydrops (n = 1), cerebral periventricular echogenicity (n = 1), and the association of two or more fetal abnormalities (hyperechogenic bowel and cerebral ventriculomegaly, n = 3).
Hepatomegaly and hepatic calcifications may be also found in cases of fetal infection. Hydrops and ascites have been documented and may be related to hepatic dysfunction and portal hypertension from liver congestion. Supraventricular tachycardia and pericardial effusion have also been reported in association with CMV infection. During a 6-year study period, Gonce et al.18 diagnosed 19 fetuses with CMV infection. The main sonographic findings were the following: brain abnormalities (n = 14), fetal hydrops (n = 4), hyperechogenic bowel (n = 4), pericardial effusion (n = 1), cardiomegaly (n = 1), oligohydramnios (n = 4), and placentomegaly (n = 2).
Although ultrasound is a valuable tool in evaluating a fetus with CMV infection, its limitations should be discussed when counseling patients. Guerra et al.17 studied the effectiveness of ultrasound in the antenatal detection of symptomatic congenital CMV infection. Six hundred and fifty ultrasound examinations of fetuses from mothers with primary CMV infection were correlated to fetal or neonatal outcome. Ultrasound abnormalities were found in 51 of 600 mothers with primary infection (8.5%) and 23 of 154 congenitally infected fetuses (14.9%). They concluded that ultrasound abnormalities predict symptomatic congenital infection in only a third of cases. Liesnard et al.9 reported characteristic sonographic findings in 9 of 55 infected fetuses (16.4%), but the majority of infected newborns were asymptomatic. Another issue is that abnormal sonographic findings may be only detected for the first time during the third trimester after a normal ultrasound examination earlier in the pregnancy. Although repeat ultrasound examination during the third-trimester scan may lead to a more accurate diagnosis and help with counseling, pregnancy termination would not be an option due to advanced gestational age.
Management: The best prevention for congenital CMV infection is primary prevention with personal hygiene practices, such as hand-washing and avoiding intimate contact with salivary secretions and urine from young children. Vaccination to CMV is currently under investigation and is not available for clinical use yet.
Although there are several methods for the prenatal diagnosis of congenital CMV infection, there is no effective treatment to offer once a diagnosis has been made. The use of CMV-specific hyperimmune globulin for treatment was evaluated by Nigro et al.19 in 157 pregnant women with primary CMV infection. Forty-five women who had a primary infection longer than 6 weeks and congenital infection confirmed by amniocentesis were enrolled. Thirty-one of these women received intravenous treatment with CMV-specific hyperimmune globulin (200 U per kg of maternal body weight), and only one had an infant with clinical CMV disease at birth. In comparison, of the 14 women who declined treatment, 7 had infants who were symptomatic at delivery (adjusted OR, 0.02, P < .001). The maternal administration of valaciclovir and ganciclovir to treat intrauterine CMV infection has also been reported.20,21 Although these studies were not randomized, they are promising and offer a possible treatment option for congenital CMV infection.
Intrauterine therapy with cytomegalovirus hyperimmunoglobulin has also been attempted. Negishi et al.22 was the first to report intraperitoneal CMV hyperimmunoglobulin administration in a fetus at 28 and 29 weeks of pregnancy. Since then, other investigators have reported the use of intraperitoneal CMV hyperimmunoglobulin as a possible treatment alternative for congenital CMV.23–25 In addition, both the intraumbilical and intra-amniotic fluid administration of CMV-specific hyperimmune globulin has been performed.26
Parvovirus
Definition and Incidence: Human parvovirus B19 is a small single-stranded DNA virus from the Parvoviridae family that is responsible for erythema infectiosum, also known as fifth disease, and is a common childhood illness. It is a worldwide infection that affects individuals from infancy through adulthood. Infection can occur at any age but most commonly affects children from 6 to 10 years of age. The prevalence of parvovirus IgG antibodies steadily rises throughout life. In children aged from 1 to 5 years and from 6 to 19 years, the prevalence of IgG antibodies is 2% to 15% and 15% to 60%, respectively. In the geriatric population, the prevalence is more than 85%.27,28
More than half of reproductive-age women have developed immunity to parvovirus B19. About 35% to 45% of women of childbearing age, however, do not have protective IgG antibodies against parvovirus. During pregnancy, the risk of acquiring parvovirus infection is low. The incidence of acute infection in pregnancy is approximately 1% to 2% during endemic periods.29 Women at increased risk include mothers of preschool and school-age children, workers at day-care centers, and school teachers. Vertical transmission occurs in about 30% to 50% of mothers infected with parvovirus during pregnancy.30 Congenital infection can cause severe fetal consequences, such as anemia, nonimmune hydrops fetalis (NIHF), and fetal death. The risk of adverse fetal outcome is increased if maternal infection occurs during the first two trimesters of pregnancy; however, fetal infection can still occur during the third trimester.31–33 It is highly unlikely that fetal infection will occur if the mother has IgG antibodies, since prior infection with parvovirus B19 confers lifelong immunity.34
Pathogenesis: The risk of fetal complications depends upon the gestational age at the time of maternal infection. The incidence of fetal morbidity and mortality decreases with gestational age. The highest risk for fetal loss happens when maternal infection develops during the 9th through the 16th weeks of pregnancy.35,36 The risk of vertical transmission is maximal at the time IgM antibodies appear. This coincides with maternal peak viral load which occurs generally 7 days after maternal inoculation.37
The virus is spread by respiratory droplets, hand-to-mouth contact and by blood products containing factor XIII and IX concentrates.31–33,38,39 Outbreaks usually occur during the spring every 4 to 5 years and may last up to 6 months. Viremia occurs 4 to 14 days after exposure and may last up to 20 days. Serum and respiratory secretions become positive for parvovirus DNA 5 to 10 days after intranasal inoculation. Symptoms such as erythema infectiosum, mild fever, arthralgias, and headaches start approximately 10 to 14 days after infection; however, many people remain asymptomatic. By the time erythema infectiosum develops, the person is usually no longer infectious.
The fetal liver, the main site of erythrocyte production, is the virus’s main target of infection.31 The fetus is more vulnerable during the second trimester when the liver is the main source of hematopoietic activity, and the half-life of red blood cells is short. Parvovirus is a potent inhibitor of hematopoiesis because it infects erythroid precursor cells such as erythroblasts and megakaryocytes. Resultant severe anemia may occur, leading to congestive heart failure and the development of hydrops fetalis. Parvovirus may infect fetal cardiac myocytes and hepatocytes, resulting in myocarditis and impaired hepatic function, respectively.40 Subsequent development of fetal high-output cardiac failure, generalized edema, and death may occur.40 Thus, the development of hydrops may not correlate with the severity of fetal anemia. Placental trophoblastic cells also express the P antigen, the main cellular receptor for the virus, and are thus susceptible to infection by parvovirus. Poor fetal outcomes have been associated with placental villous trophoblast apoptosis in patients with congenital infection.41 Such observations suggest that parvovirus infection may be associated with placental insufficiency. Stillbirth in nonhydropic fetuses may result from placental damage and occurs in 0.9% to 23% of pregnancies with documented maternal infection.42,43
Diagnosis: Serologic examination of maternal blood is the initial and most useful diagnostic tool for parvovirus. Specific IgM and IgG testing should be performed as soon as possible once maternal infection is suspected during pregnancy.44 IgM antibodies become detectable in maternal serum within 7 to 10 days after infection, sharply peak at 10 to 14 days, and can persist in the circulation for 3 to 4 months or longer.45 IgG antibodies will rise considerably more slowly and reach a plateau at 4 weeks after infection. Of note, after a recent contact, there will be a serologic window of 7 days, during which both IgG and IgM remain undetectable.44 Women who are IgG-positive and IgM-negative can be reassured that there is no evidence of recent infection. Patients with IgG- and IgM-negative–specific antibody should be considered susceptible, and further serological testing should be carried out 4 weeks after the last contact or if signs of the disease develop.35 IgM-positive patients, irrespective of IgG status, should receive serial fetal evaluation to rule out congenital infection.
Since most infected pregnancies have a favorable outcome, invasive prenatal diagnostic testing should only be used if there are definitive signs of fetal anemia or hydrops fetalis.35 Ultrasound must be used for diagnosis and surveillance. Fetal infection may be identified by using PCR of parvovirus viral DNA in amniotic fluid or fetal cord blood. Amniocentesis is the preferred method of choice due to less complications and increased availability. Serologic examination of fetal blood samples is highly unreliable since the IgG and IgM response to parvovirus is not produced during intrauterine life. Detection of parvovirus using specific IgM in fetal blood has a sensitivity of 29% compared to almost 100% for PCR.35,44,46
Ultrasound: As soon as a recent parvovirus maternal infection is suspected during pregnancy, ultrasound examination should be performed to exclude the presence of fetal anemia and hydrops. The virus infects the liver which is the main site of erythrocyte production in the fetus leading to anemia, most often occurring during the second trimester. Increased cardiac output and decreased viscosity of fetal blood caused by anemia are responsible for the changes in fetal blood during congenital infection. An increase in the middle cerebral artery peak systolic velocity (MCA-PSV) (Fig. 23.5) is a very sensitive measure to identify fetal anemia caused by parvovirus infection.47 Weekly measurements of MCA-PSV are recommended after maternal infection is documented. Timing of intrauterine transfusion for treatment of fetal anemia and prevention of fetal hydrops can be based on these MCA-PSV measurements. The infection causes anemia and possible myocarditis, leading to high-output cardiac failure and subsequent development of generalized edema. Fetal hydrops, an accumulation of excess fluid in at least two body compartments of the fetus, can be easily seen with ultrasound. The median interval between maternal parvovirus infection and diagnosis of hydrops fetalis is about 3 weeks, but may vary between 1 and 20 weeks.48 The ultrasonographic findings include fetal ascites, skin edema, pericardial effusion, pleural effusions, and placental edema (Figs. 23.6 to 23.11). Enlargement and thickening of the fetal heart also may be documented during the examination. Fetal structural anomalies associated with parvovirus are uncommon; however, ultrasound findings such as hydrocephalus, hyperechogenic bowel, meconium peritonitis, fetal liver calcifications, cleft lip and palate, and increased fetal nuchal translucency have been reported.48–52
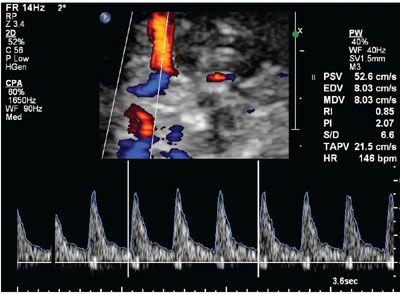
FIGURE 23.5: Duplex Doppler of the middle cerebral artery at 24 gestational weeks demonstrating increased peak systolic velocity in a fetus exposed to parvovirus.
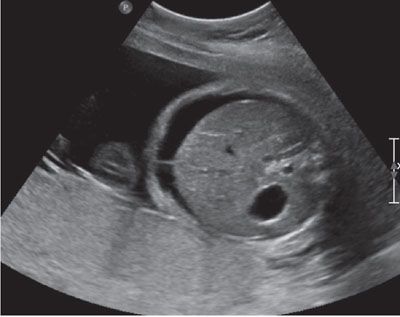
FIGURE 23.6: Cross-sectional ultrasound view of the abdomen showing marked ascites in a fetus with congenital parvovirus.
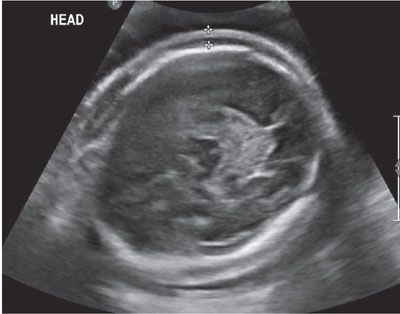
FIGURE 23.7: Axial ultrasound image of the fetal head showing scalp edema in a pregnancy exposed to parvovirus and positive IgM titers.
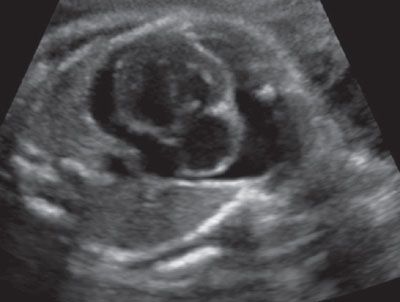
FIGURE 23.8: Cross-sectional ultrasound view of the fetal chest demonstrating marked pericardial effusion in a pregnancy with confirmed parvovirus infection by amniotic fluid PCR.
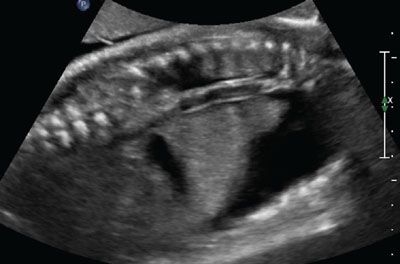
FIGURE 23.9: Sagittal view of chest demonstrating pleural effusion at 20 gestational weeks in a fetus with congenital parvovirus.
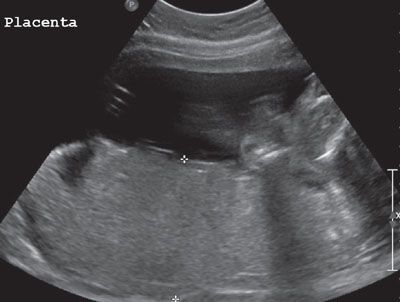
FIGURE 23.10: Placentomegaly demonstrated by ultrasound in a pregnancy complicated by fetal parvovirus infection.
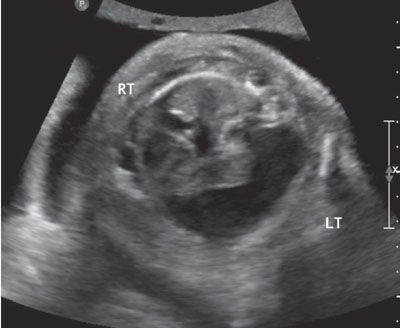
FIGURE 23.11: Cross-sectional view of the chest in a fetus with congenital parvovirus showing bilateral pleural effusions, more prominent in the left hemithorax. The fetal heart is displaced to the right side of the chest.
Management: Maternal infection with parvovirus is self-limited and is treated symptomatically. Acute red cell aplasia may rarely occur and requires serial measurements of hemoglobin and possible blood transfusions to prevent maternal complications due to severe anemia. The confirmation of fetal infection is not required for suspected maternal infection.
If serologic evidence suggests possible maternal infection, weekly measurements of fetal MCA-PSV and US assessment for hydrops should be performed. The MCA-PSV may diagnose significant fetal anemia with a sensitivity of as high as 100%.53 In parvovirus infection, the MCA-PSV was reported to have a sensitivity of 94.1% for the diagnosis of fetal anemia.47 Percutaneous umbilical blood sampling (PUBS) should be considered once the MCA-PSV reaches 1.5 multiples of the median (MOM) for gestational age. If fetal anemia is confirmed, fetal blood transfusion is indicated. Intrauterine transfusion with packed red blood cells for cases of severe fetal anemia has been shown to reduce perinatal morbidity and mortality. Fetal survival may be as high as 60% to 80% when intrauterine transfusion is attempted, compared to only 15% to 30% with hydrops and no intervention. Fairley et al.54 compared outcomes of expectant management with intrauterine transfusion in cases of maternal parvovirus infection, and found a greater than 7-fold reduction in fetal death with the use of intrauterine transfusion.
In addition, a targeted fetal ultrasound and echocardiography should be performed, especially in cases complicated by hydrops fetalis. Delivery is recommended at 34 gestational weeks in pregnancies affected with hydrops fetalis. An attempt to correct fetal anemia should be considered before delivery to improve neonatal outcome.
Prognosis: The long-term prognosis for children who received intrauterine transfusion (IUT) for congenital parvovirus infection is controversial. Some studies have shown that children who underwent a successful intrauterine transfusion have good neurodevelopmental outcomes.36,55 Miller et al.36 described seven cases of fetal hydrops, two of which received IUT. They were unable to find any long-term developmental problems in the patients who received fetal blood transfusion. Similarly, Dembinski et al.55 followed up 20 children with parvovirus infection treated with IUT and found no evidence of developmental delay. On the other hand, de Jong et al.44 described an increased risk of both neurodevelopmental delay and cerebral palsy in children treated with intrauterine transfusions for congenital parvovirus infection. Parvovirus DNA has been detected within white matter multinucleated, reactive microglial cells, suggesting that the virus itself may play a direct role in perivascular changes and white matter damage.56 Alternatively, severe fetal anemia and hydrops may cause hypoxic-ischemic cerebral injury, thereby, contributing to the increased rate of neurodevelopmental complications. Lindenburg et al.57 demonstrated similar findings of developmental delay and cerebral palsy in neonates that underwent IUT for severe fetal anemia. However, for a number of reasons, these fetuses are likely to be delivered prematurely, which is itself a significant risk factor for pediatric developmental disorders.
Rubella Virus
Definition and Incidence: Rubella, also known as German measles and “third” disease, is caused by a lipid-enveloped, single-stranded RNA togavirus.58 Congenital rubella syndrome (CRS) was one of the earliest-described vertically transmitted infections in the newborn infant.59 The last major epidemic of rubella in the United States occurred between 1964 and 1965, during which time 20,000 cases of infants were born with CRS. Cases of infection during pregnancy have been significantly reduced following vaccine development in 1969. From 1995 to 2000, an average of five cases of CRS has been reported annually in the United States. Newborns affected by CRS are most commonly born in countries where routine rubella vaccination programs are not used.
Pathogenesis: Rubella is an airborne transmitted infection spread by small respiratory droplets which becomes infectious 7 days prior to the appearance of the initial symptoms. Vertical transmission is hypothesized to occur 5 to 7 days following maternal inoculation.60 The clinical features of rubella are a rash, fever, arthralgias, and lymphadenopathy. The rash generally manifests initially on the face and then gradually migrates toward the trunk and then to the lower extremities.60 Generally self-limited complications such as encephalitis, thrombocytopenia, neuritis, conjunctivitis, and orchitis have rarely been reported to occur as a result of rubella.60–62 Importantly, encephalitis from rubella infection has been associated with a 50% mortality. Subclinical infection can occur in up to 50% of patients; however, in these patients, fetal anomalies as a result of congenital infection rarely occur. Reinfection with rubella after prior documented infection or immunization is extremely rare during pregnancy.63,64 Antibody titers lower than 1/64 has been associated with reinfection.65 The risk of vertical transmission depends upon gestational age and is 90%, 25%, and 95% during the first, second, and third trimesters, respectively. During the first trimester, CRS is extremely rare if the maternal rash occurs within the first 2 gestational weeks. If the rash appears during the 3rd gestational week, the infection rate is 31%, and nearly 100% afterwards.66 Congenital heart defects and deafness most commonly occur in infected fetuses during the first trimester, but are rare afterwards.
Laboratory Studies: Serological analysis is based on the detection of IgG, IgM, and also IgG avidity antibodies.60,67 The enzyme-linked immunosorbent assays (ELISA), hemoagglutination inhibition test (HI), and the immunofluorescent antibody assay (IFA) are the most common methods of antibody detection.59,60,67–69 Acute rubella infection is characterized by the appearance of rubella IgM about 5 days after the onset of the maternal rash and persists for 6 weeks.70 The presence of IgM antibodies does not always correspond to an acute infection. A false-positive IgM for rubella can result in patients with parvovirus, mononucleosis, or a positive rheumatoid factor.71 In addition, IgM antibodies may persist for 1 year or more in a chronic rubella carrier. Thus, in order to properly establish a timeline of infection, it is important to measure the Rubella IgG avidity. A high IgG avidity indicates chronic carrier status, while a lower IgG avidity indicates a more recent infection. The evaluation of IgG, IgM, or the RNA virus in the saliva instead of in the blood has been proposed to diagnose rubella.72–75 Ramsay et al.75 found a sensitivity of 98% and a specificity of 100% for IgG, and a specificity of 99% for IgM detected in the saliva.
Amniotic fluid sampling for fetal diagnosis of CRS should be performed 6 to 8 weeks after maternal infection to avoid false-negative results. Samples of amniotic fluid may be sent for viral cultures; however, this method lacks sensitivity and final results may take up to 6 weeks. PCR technology may be used to diagnose CRS, having the advantage of increased detection rates and faster result times.69 The diagnosis of CRS via chorionic villus sampling has been described.76
Ultrasound: Ultrasound has an important role in the prenatal diagnosis of CRS. Migliucci et al.77 performed a retrospective study on 175 women referred for rubella infection. Sonographic findings of IUGR, polyhydramnios, cardiomegaly, atrial septal defect, hepatosplenomegaly, ascites, echogenic bowel, and placentomegaly were detected. Ugurbas et al.78 reported an association between microphthalmos and CRS. Ventricular septal defect and pulmonary stenosis have also been reported in cases of CRS.79 Exencephaly was diagnosed in one case.80
Management: Maternal rubella is a self-limited disease requiring only symptomatologic care. Severe complications of rubella infection such as encephalitis, thrombocytopenia, neuritis, conjunctivitis, and orchitis should be aggressively managed. Termination of pregnancy may be offered in cases of maternal infection. The maternal administration of immune globulin in large doses (20 mL in adults) in cases of susceptible women exposed to rubella during gestation has been proposed.67 This treatment, however, has not produced encouraging results because it does not seem to prevent fetal infection. Post exposure prophylaxis for rubella in early pregnancy is not recommended due to unproven clinical efficacy. The rubella vaccine is contraindicated during pregnancy; therefore, susceptible women should be immunized postpartum.
Prognosis: Up to two-thirds of children with congenital rubella may be asymptomatic at birth, but will develop sequelae within the first 5 years of life.74 Classic findings associated with neonatal rubella are low birth weight with a cluster of abnormalities, including cataracts, sensorineural deafness, and cardiac defects such as patent ductus arteriosus, pulmonary artery stenosis, and coarctation of aorta. Less common neonatal heart defects that have been reported are aortic stenosis and Ebstein anomaly.81–84 Purpura (blueberry muffin spots), microphthalmia, corneal opacity, glaucoma, hepatosplenomegaly, thrombocytopenia, and radiolucent bone lesions may also be found.85 Late manifestations of congenital rubella include hearing loss, pancreatic insufficiency, and behavioral disorders.74,85 Diagnosis is made by serum detection of rubella IgM before 3 months of age or persistent IgG between 6 and 12 months of age.
Herpes Simplex Virus
Definition and Incidence: Genital HSV type 1 (HSV-1) or HSV type 2 (HSV-2) is a common infection in United States, affecting 16.2% or 1 in 6 people between the ages of 14 and 49 years.86 Approximately 25% to 65% of pregnant patients in the United States have genital infection with HSV. The frequency of neonatal HSV infection in the United States varies according to the patient population, with the rate of infection ranging from 1 case per 12,500 to 1 case per 1,700 live births. Whitley et al.87 analyzed the data from 30 U.S. health plans and showed a rate of 60 cases per 100,000 live births. This incidence is higher than that of congenital syphilis, toxoplasmosis, and congenital rubella.
Pathogenesis: HSV belongs to the family of double-stranded DNA viruses known as Alphaherpesvirinae, a subfamily of the Herpesviridae. HSV type 1 (HSV-1) and type 2 (HSV-2) are differentiated based on the glycoproteins within the lipid envelope. Glycoproteins G1 and G2 are associated with HSV-1 and HSV-2, respectively. The hallmark of herpes infection is the ability to infect epithelial mucosal cells where replication occurs. The virus, then, gains access to sensory neurons and stay latent in the sensory ganglia for years, followed by reactivation.
HSV is transmitted from person to person through direct contact. HSV-1 is usually acquired orally, but may also be sexually transmitted. HSV-2 is primarily a sexually transmitted infection. Most neonatal infections result from exposure to HSV in the genital tract during delivery, although both viruses may also be transmitted vertically during pregnancy. Traditionally, HSV-1 was typically associated with orofacial lesions while HSV-2 was felt to cause genital herpes. Although HSV-2 still predominates as the major etiology for genital herpes, an increasing proportion has been ascribed to HSV-1 recently, especially in younger women. According to the Centers for Disease Control and Prevention (CDC), the rate of HSV-2 seroprevalence in the United States has remained stable since the mid-1990s at 16.2%.86
The three categories of genital herpes infections are primary, nonprimary, and recurrent. A primary HSV infection is a newly acquired infection in the absence of preexisting antibodies to either HSV-1 or HSV-2. Primary symptomatic genital herpes have an incubation of a period of 2 to 20 days and cause ulceration of the external genitalia and cervix as well as blistering lesions on the internal thigh, buttocks, and perineal skin. Primary HSV infections can also be associated with a number of systemic symptoms such as fever, malaise, and headache.
Nonprimary episode infection refers to newly acquired antibodies to HSV-1 or 2 in the presence of preexisting antibodies to the other type. Nonprimary infections tend to be less severe than primary HSV infections and to have less systemic symptoms and quicker recovery times. HSV-2 antibodies are highly protective against new HSV-1 infection, thus, nonprimary HSV-1 infections are much less common.
Recurrent genital HSV infections refer to the reactivation of a latent genital HSV. The HSV type obtained from the lesion matches the HSV type obtained from the serum. Recurrent infections are typically less severe, unilateral, and have fewer lesions than either primary or nonprimary infections.88 As in nonprimary HSV, recurrent genital HSV infections are more common with HSV-2 as opposed to HSV-1. Asymptomatic viral shedding may occur during phases in between clinical outbreaks of genital herpes, where HSV reactivates within the sensory neurons of the genital mucosa. Most sexual transmission of HSV occurs during periods of asymptomatic viral shedding because patients are unaware that they are infectious.89 Most cases of genital HSV infection in women occur without signs or symptoms of disease and are associated with cervical viral shedding.
Diagnosis: There are a variety of methodologies for the diagnosis of HSV infection, including viral culture, PCR, direct fluorescent antibodies, Tzanck smears, and serologic identification of IgG and IgM. Pregnant women who present with symptoms suggestive of genital herpes should undergo both type-specific assay and viral identification testing. Routine antepartum screening in asymptomatic patients is not recommended.
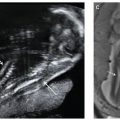
Ultrasound: Since intrauterine HSV infection is very uncommon, limited experience exists regarding the sonographic prenatal diagnosis. Various fetal malformations have been associated with congenital herpes, including microcephaly, cerebral atrophy, hydranencephaly, intracranial calcifications, ventriculomegaly, microphthalmia, chorioretinitis, cataracts, congenital herpetic keratitis, congenital heart disease, hepatic calcifications, nonimmune hydrops, bullous skin lesions and scars, lower-limb hypoplasia, and abnormal digits. Fetal cerebral malformation, echogenic bowel (Fig. 23.12), and skin lesions seem to be more common ultrasound findings in fetuses with congenital herpes. Brain lesions are considered as secondary to the cytotoxic virus effects, or subsequent ischemia caused by vascular occlusion of cerebral vessels. Lanouette et al.90 reported a 14-fold increase in α-fetoprotein (AFP) noted during second trimester screening in a patient with multiple fetal congenital anomalies. At 19 weeks’ gestation, the patient underwent an amniocentesis and cordocentesis with results consistent with HSV infection.
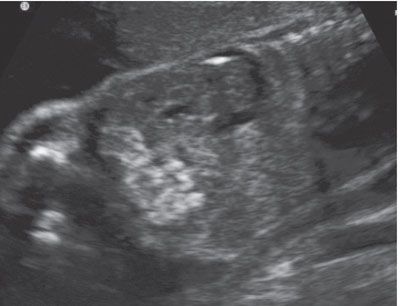
FIGURE 23.12: Sagittal-oblique ultrasound view demonstrating echogenic bowel at 26 gestational weeks in a fetus with congenital herpes simplex virus.
Jayaram and Wake91 reported a case with confirmed maternal HSV-2 infection in which a screening ultrasound at 20 weeks did not show any abnormalities. During the third trimester, the patient was admitted with reduced fetal movements. An ultrasound examination then noted absent corpus callosum and gross ventriculomegaly associated with absent end-diastolic flow of umbilical artery Doppler. Interestingly, an irregular heart rate with fluctuating baseline between 60 and 160 beats per minute was also documented.
In a case report by Diguet et al.,92 during a screening ultrasound at 23 weeks of gestation, IUGR, absence of limb movements, thickened skin, hyperechogenic bowel, placental micronodular alterations, moderate pericardial effusion, and a reverse flow of the ductus venosus were noted. Because of a previous clinical episode of HSV at the beginning of pregnancy, amniocentesis was performed, revealing a positive PCR for HSV-1. On follow-up sonographic examination at 27 weeks of gestation, oligohydramnios associated with fetal abdominal and lower-limb skin irregular thickness, esophageal hyperechogenicity, and persistence of IUGR were found. The pregnancy was terminated, and fetal examination revealed extensive skin ulceration on the trunk and limbs, splenomegaly, and cardiomegaly.
Duin et al.93 reported a case in a patient with confirmed HSV in which a normal ultrasound at 20 weeks’ gestation with symmetric fetal growth was obtained; however, during a third-trimester examination, marked cerebral ventriculomegaly, third ventricle enlargement, frontal thinning of the cerebral cortex, and microcephaly were noted. A follow-up ultrasound demonstrated a slight dissolution of the cortical mantle. Severe parenchymal destruction, particularly in the temporal and parietal lobes, was confirmed by a prenatal MRI. The occipital cerebral cortex was also globally thinned with microgyria. Postmortem examination confirmed the prenatal diagnosis of hydranencephaly. This report suggests that fetal MRI may provide significant additional information in assessing the extensiveness of the fetal HSV infection. Interestingly, fetal malformation caused by congenital herpes infection may be only evident during the late part of pregnancy despite an initial normal screening ultrasound. Thus, third-trimester ultrasound evaluation for an anatomy follow-up and biometry should be considered in pregnancies with known herpes virus infection.
Management: Although considerable effort is made for the viral identification of genital HSV, treatment regiments do not vary by virus type. During a primary outbreak in pregnancy, oral antibiotic therapy is indicated to reduce the duration and severity of symptoms. Viral shedding is also decreased with proper therapy. No data indicates that maternal treatment reduces the risk of neonatal herpes. Acyclovir is not teratogenic and may be administered either orally in pregnant women with a first episode of genital herpes or intravenously in pregnant women with severe genital or disseminated herpetic disease.
Transabdominal invasive procedures, such as chorionic villus sampling, amniocentesis, and percutaneous umbilical sampling, may be performed even when genital lesions are present. Transcervical procedures should not be performed during the presence of active lesions.
Prognosis: Neonatal HSV infection is defined as infection in a newborn within 28 days after birth. There are three categories of neonatal infections: cutaneous disease, CNS disease, and disseminated disease. Cutaneous disease is a HSV disease localized to the skin, eye, and/or mouth. Although cutaneous disease has a low mortality, it may progress to CNS or disseminated disease. CNS disease manifests with neurologic symptoms as well as positive CSF PCR findings and carries a mortality of approximately 15%. Disseminated disease has the worst prognosis with the highest fatality rate. Involvement of multiple organs (e.g., hepatitis, pneumonitis, or disseminated intravascular coagulation) is common and has a mortality rate of 31% and 85%, with and without therapy, respectively.
Human Immunodeficiency Virus
Definition and Incidence: Acquired immune deficiency syndrome (AIDS) is a severe immunological disorder caused by the HIV RNA retrovirus, resulting in a defect in cell-mediated immune response leading to an increased susceptibility to opportunistic infections. Despite aggressive efforts by the health community to reduce vertical transmission, HIV remains a significant perinatal risk globally. Almost 33.3 million people worldwide are infected, with 88% of infected infants born to mothers who did not receive any antiretroviral treatment.94,95 In the United States, approximately 21% of patients with HIV are unaware of their infection96; so the CDC recommends routine preconceptional HIV-testing for all women.97
Pathogenesis: HIV attaches to the CD4 molecule on T lymphocytes via the external glycoprotein (gp120) and the transmembrane protein (gp41) located on the HIV envelope.98
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree