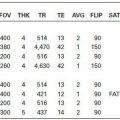
KEY FACTS
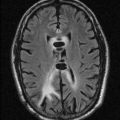
 Accounts for 20% ofallhydrocephalus cases; generally presents in infancy but may manifest at any time.
Accounts for 20% ofallhydrocephalus cases; generally presents in infancy but may manifest at any time.
 This spectrum of disorders includes congenital narrowing of the aqueduct (septum or membrane, forking, gliosis, webs, or stenosis), postinflammatory changes (post meningitis, post hemorrhage), and tumors (especially those arising in the tectum).
This spectrum of disorders includes congenital narrowing of the aqueduct (septum or membrane, forking, gliosis, webs, or stenosis), postinflammatory changes (post meningitis, post hemorrhage), and tumors (especially those arising in the tectum).
 Causes deformity of tectum, which appears thick but not bulbous (if so, consider tumor that is almost always bright on T2/fluid-attenuated inversion recovery [FLAIR] images). High-resolution T2 images (constructive interference in steady state [CISS] or FIESTA) are useful in identifying aqueductal abnormality; flow-sensitive images confirm diagnosis.
Causes deformity of tectum, which appears thick but not bulbous (if so, consider tumor that is almost always bright on T2/fluid-attenuated inversion recovery [FLAIR] images). High-resolution T2 images (constructive interference in steady state [CISS] or FIESTA) are useful in identifying aqueductal abnormality; flow-sensitive images confirm diagnosis.
 May be associated with Chiari type I and II, Dandy-Walker, and other congenital malformations.
May be associated with Chiari type I and II, Dandy-Walker, and other congenital malformations.
 Endocrine dysfunction occurs in 15% to 20% of patients and is probably secondary to compression of hypothalamus-pituitary axis due to enlarged third ventricular recesses.
Endocrine dysfunction occurs in 15% to 20% of patients and is probably secondary to compression of hypothalamus-pituitary axis due to enlarged third ventricular recesses.
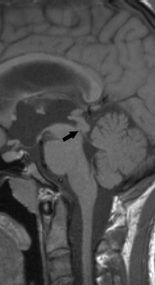
FIGURE 19-1. Midsagittal T1 image shows distal narrowing (arrow) of aqueduct.

FIGURE 19-2. Midsagittal flow-sensitive study, in a different patient, shows cerebrospinal fluid (CSF) flow in black that is present only in the superior aspect of aqueduct and stops suddenly (arrow).
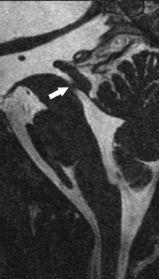
FIGURE 19-3. Midsagittal high-resolution CISS mage, in a different patient, shows a web (arrow) in the distal aqueduct.
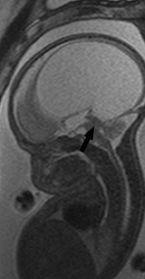
FIGURE 19-4. Midsagittal T2 in utero image shows aqueductal stenosis (arrow) and massive hydrocephalus.
SUGGESTED READING
Stoquart-El Sankari S, Lehmann E Gondry-Jouet C, Fichten A, Godefroy O, Meyer M-E, Baledent O. Phase-contrast MR imaging support for the diagnosis of aqueductal. Stenosis. Am J Neuroradiol 2009;30:209–214.
KEY FACTS
 Found more often in adults (incidentally by magnetic resonance imaging [MRI] ) than in children.
Found more often in adults (incidentally by magnetic resonance imaging [MRI] ) than in children.
 Etiology: probably related to mesenchymal malformation of craniocervical junction leading to hypoplasia of bones and dura.
Etiology: probably related to mesenchymal malformation of craniocervical junction leading to hypoplasia of bones and dura.
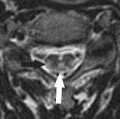
 Defined as displacement of cerebellar tonsils below (>6 mm) the foramen magnum, which in itself is relatively small; 5% to 30% of patients with this degree of displacement are symptomatic; patients with displacement > 12 mm are always symptomatic.
Defined as displacement of cerebellar tonsils below (>6 mm) the foramen magnum, which in itself is relatively small; 5% to 30% of patients with this degree of displacement are symptomatic; patients with displacement > 12 mm are always symptomatic.
 Displacement of tonsils between 3 and 6 mm is indeterminate; <3 mm is normal. Cerebellar tonsils need to be “pointed” inferiorly to make diagnosis in mild cases.
Displacement of tonsils between 3 and 6 mm is indeterminate; <3 mm is normal. Cerebellar tonsils need to be “pointed” inferiorly to make diagnosis in mild cases.
 CSF flow studies shows lack of flow posteriorly or around the foramen magnum and a small or absent cisterna magna. Movement of tonsils on CSF flow studies is also abnormal.
CSF flow studies shows lack of flow posteriorly or around the foramen magnum and a small or absent cisterna magna. Movement of tonsils on CSF flow studies is also abnormal.
 Symptoms: headache (hydrocephalus in 25% of cases), neck pain, nystagmus, lower cranial nerve palsies, basilar invagination (25%), odontoid deformities, scoliosis, spinal cord cysts (20% to 40%), Klippel-Feil syndrome, and atlanto-occipital assimilation.
Symptoms: headache (hydrocephalus in 25% of cases), neck pain, nystagmus, lower cranial nerve palsies, basilar invagination (25%), odontoid deformities, scoliosis, spinal cord cysts (20% to 40%), Klippel-Feil syndrome, and atlanto-occipital assimilation.
 Spinal cord cysts are more common in the cervical region.
Spinal cord cysts are more common in the cervical region.
 Treatment generally involves resection of the hypoplastic posterior arch of Cl, durai resection with duroplasty, and recreation of cisterna magna; some surgeons resect the cerebellar tonsils.
Treatment generally involves resection of the hypoplastic posterior arch of Cl, durai resection with duroplasty, and recreation of cisterna magna; some surgeons resect the cerebellar tonsils.
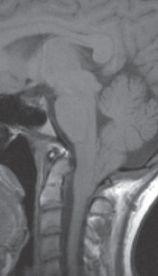
FIGURE 19-5. Midsagittal T1 image shows marked inferior displacement of triangular-shaped cerebellar tonsils.
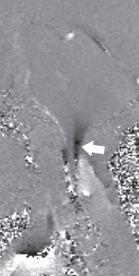
FIGURE 19-6. CSF flow study shows significant inferior motion of tonsils (arrow) and abnormal flow anterior to cord.

FIGURE 19-7. CSF flow study, in a different patient, shows normal cephalad flow anterior in white (arrow) but absent flow posteriorly.
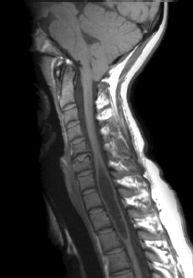
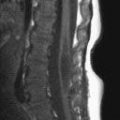
FIGURE 19-8. Midsagittal T1 image shows cerebellar tonsil herniation and midcervical cord cyst.
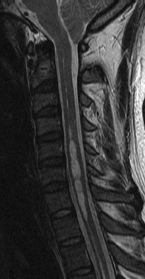
FIGURE 19-9. Midsagittal T2 image shows tonsillar herniation, absent cisterna magna, and cord cyst.
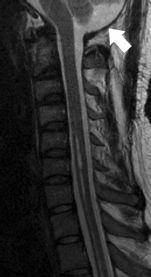
FIGURE 19-10. The same patient as 19-9 after surgery shows recreation of cisterna magna (arrow), resection of tonsils, and near resolution of cord cyst.
SUGGESTED READING
Schijman E. History, anatomic forms, and pathogenesis of Chiari I malformations. Childs Nerv Syst 2004;20(5): 323–328.
Dolar MT, Haughton VM, Iskandar BJ, Quigley M. Effect of craniocervical decompression on peak CSF velocities in symptomatic patients with Chiari I malformation. Am J Neuroradiol 2004;25:142–145.
KEY FACTS
 Complex anomaly always associated with myelomeningocele (chronic CSF leakage in utero may lead to collapse of developing brain, producing Chiari II changes).
Complex anomaly always associated with myelomeningocele (chronic CSF leakage in utero may lead to collapse of developing brain, producing Chiari II changes).
 Skull and dura anomalies include lacunar skull (resolves spontaneously by 6 to 12 months), scalloped petrous ridges and clivus, large foramen magnum, insufficient tentorial incisura, and hypoplastic or fenestrated falx.
Skull and dura anomalies include lacunar skull (resolves spontaneously by 6 to 12 months), scalloped petrous ridges and clivus, large foramen magnum, insufficient tentorial incisura, and hypoplastic or fenestrated falx.
 Brain anomalies: interiorly displaced vermis into the foramen magnum, heart-shaped cerebellum displaced superiorly through insufficient tentorial incisura, beaked tectum, callosal agenesis, interdigitation of cortical sulci in superior midline, and anomalies of neuronal migration.
Brain anomalies: interiorly displaced vermis into the foramen magnum, heart-shaped cerebellum displaced superiorly through insufficient tentorial incisura, beaked tectum, callosal agenesis, interdigitation of cortical sulci in superior midline, and anomalies of neuronal migration.
 Brain anomalies may be subtle after in utero treatment of spine dysraphism.
Brain anomalies may be subtle after in utero treatment of spine dysraphism.
 About 90% of patients have hydrocephalus and colpocephaly (dilatation of atria and occipital horns of lateral ventricles).
About 90% of patients have hydrocephalus and colpocephaly (dilatation of atria and occipital horns of lateral ventricles).
 Spinal cord cysts are seen in up to 90% of patients.
Spinal cord cysts are seen in up to 90% of patients.
 Segmentation anomalies of the upper cervical spine are seen in 10% of patients.
Segmentation anomalies of the upper cervical spine are seen in 10% of patients.
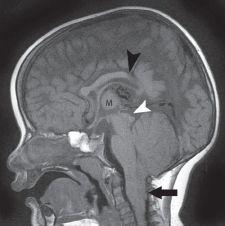
FIGURE 19-11. Midsagittal T1 image shows the dysgenetic corpus callosum (black arrowhead, posterior body/splenium are absent), “beaked” tectum (white arrowhead), large massa intermedia (M), absent aqueduct, absence of normal sulcation of cerebellar vermis, small fourth ventricle, and interiorly herniated vermis (arrow).
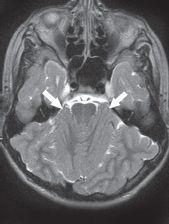
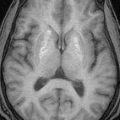
FIGURE 19-12. Axial T2, in the same patient, shows anterior location of cerebellar hemispheres (arrows).
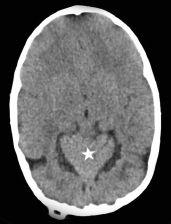
FIGURE 19-13. Axial computed tomography (CT), in a different patient, shows a “heart-shaped” (star) superiorly displaced cerebellum through a wide tentorial incisura.
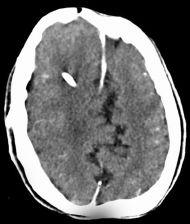
FIGURE 19-14. Axial CT, in a different patient, shows an incomplete mid-falx with “interdigitation” of cortical sulci.
SUGGESTED READING
McLone DG, Di as MS. The Chiari II malformation: cause and impact. Childs Nerv Syst 2003;19:540–550.
 POSTERIOR (OCCIPITAL AND/OR PARIETAL) ENCEPHALOCELES
POSTERIOR (OCCIPITAL AND/OR PARIETAL) ENCEPHALOCELES
KEY FACTS
 Rare malformations: 1 to 3:100,000 live births.
Rare malformations: 1 to 3:100,000 live births.
 In the North America, most encephaloceles are occipital (80%) and parietal (10%); in Asia and Latin America, most are syncipital ( frontoethmoidal); rare types include atretic parietal, sphenoi-dal (associated to neurofibromatosis), and nasopharyngeal.
In the North America, most encephaloceles are occipital (80%) and parietal (10%); in Asia and Latin America, most are syncipital ( frontoethmoidal); rare types include atretic parietal, sphenoi-dal (associated to neurofibromatosis), and nasopharyngeal.
 Herniated brain is usually nonfunctioning due to necrosis, gliosis, fibrosis, and anomalies of neuronal migration.
Herniated brain is usually nonfunctioning due to necrosis, gliosis, fibrosis, and anomalies of neuronal migration.
 Encephalocele may contain pons, midbrain, and aberrant but important venous structures.
Encephalocele may contain pons, midbrain, and aberrant but important venous structures.
 Atretic parietal meningocele refers to a form “fruste” with only a small amount of méninges, gliotic brain, and CSF in the parietal region that may cross the superior sagittal sinus and be associated with other venous anomalies (commonly a persistent falcine sinus).
Atretic parietal meningocele refers to a form “fruste” with only a small amount of méninges, gliotic brain, and CSF in the parietal region that may cross the superior sagittal sinus and be associated with other venous anomalies (commonly a persistent falcine sinus).
 Spinal cord cysts may be present.
Spinal cord cysts may be present.
 Chiari III is very rare and is the combination of the intracranial features of Chiari II with a low occipital and high cervical encephalocele.
Chiari III is very rare and is the combination of the intracranial features of Chiari II with a low occipital and high cervical encephalocele.
 Occipital/parietal encephaloceles may be associated with the Dandy-Walker malformations.
Occipital/parietal encephaloceles may be associated with the Dandy-Walker malformations.
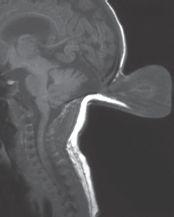
FIGURE 19-15. Midsagittal T1 shows a mostly CSF-filled occipital encephalocele and some tec-tal “beaking.”.
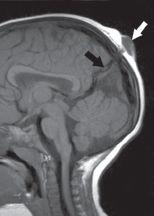
FIGURE 19-16. Midsagittal T1 shows the form “fruste” (atretic parietal meningocele, white arrow) extending through the superior sagittal sinus and associated with a persistent falcine sinus (black arrow).
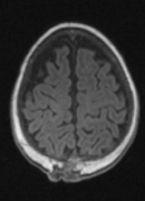
FIGURE 19-17. Axial T1, in a different patient, shows the atretic parietal meningocele.
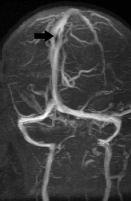
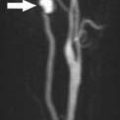
FIGURE 19-18. Oblique TOF MR venogram shows fenestration (arrow) of the superior sagittal sinus in the same patient as 19-17.
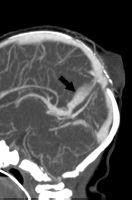
FIGURE 19-19. Midsagittal CT venogram shows persistent parietal sinus (arrow) in a patient with an atretic parietal meningocele.
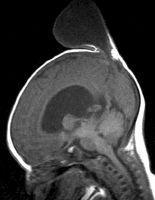
FIGURE 19-20. Midsagittal T1 shows a large parietal meningocele.
SUGGESTED READING
Caldarelli M, Rea G, Cincu R, Di Rocco C. Chiari type III malformation. Childs Nerv Syst 2002;18:207–210. Patterson RJ, Egelhoff JC, Crone KR, Ball WS Jr. Atretic parietal cephaloceles revisited: an enlarging clinical and imaging spectrum? Am J Neuroradiol 1998;19:791–795.
KEY FACTS
 The incidence of sincipital encephaloceles in the United States is low (1:20,000–40,000 live births); they are more common in Asia and Latin America.
The incidence of sincipital encephaloceles in the United States is low (1:20,000–40,000 live births); they are more common in Asia and Latin America.
 More common in boys and always accompanied by hypertelorism.
More common in boys and always accompanied by hypertelorism.
 Encephalocele locations: nasofrontal (40% to 60%), nasoethmoidal (30%), and nasolateral (also called naso-orbital).
Encephalocele locations: nasofrontal (40% to 60%), nasoethmoidal (30%), and nasolateral (also called naso-orbital).
 Most sincipital encephaloceles contain nonfunctioning gliotic brain and are accompanied by complex intracranial malformations (particularly the larger ones).
Most sincipital encephaloceles contain nonfunctioning gliotic brain and are accompanied by complex intracranial malformations (particularly the larger ones).
 Differential diagnosis includes nasal gliomas (brain heterotopias) and nasal dermoids.
Differential diagnosis includes nasal gliomas (brain heterotopias) and nasal dermoids.
 Nasal gliomas are isolated masses of brain trapped in the prenasal space; they may grow slightly, enhance after contrast, and often have signal intensities different than that of brain due to gliosis.
Nasal gliomas are isolated masses of brain trapped in the prenasal space; they may grow slightly, enhance after contrast, and often have signal intensities different than that of brain due to gliosis.
 Nasal dermoids may form in the prenasal space or outside of it anywhere from the tip of the nose to the nasal bones; they have varying signal intensities and may enhance particularly if infected; occasionally, they have a fatty nature; they tend to communicate with the foramen cecum in front of a wide or bifid crista galli.
Nasal dermoids may form in the prenasal space or outside of it anywhere from the tip of the nose to the nasal bones; they have varying signal intensities and may enhance particularly if infected; occasionally, they have a fatty nature; they tend to communicate with the foramen cecum in front of a wide or bifid crista galli.
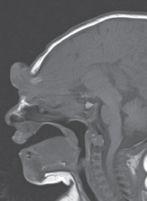
FIGURE 19-21. Midsagittal T1 shows a frontona-sal encephalocele.
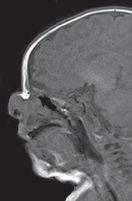
FIGURE 19-22. Midsagittal T1, in a different patient, shows an anterior nasal encephalocele and an anterior ethmoidal bone defect (arrow).
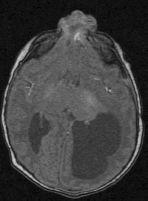
FIGURE 19-23. Axial FLAIR shows the encephaocele in the same patient as 19-22.
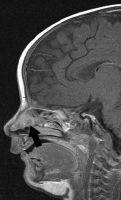
FIGURE 19-24. Midsagittal postcontrast T1, in a different patient, shows an enhancing intranasal mass (arrow) proven to be isolated brain (nasa glioma).
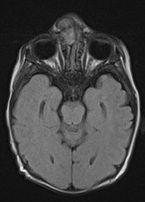
FIGURE 19-25. Axial FLAIR, in a different patient, shows an intranasal and extranasal glioma.
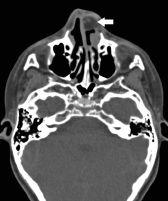
FIGURE 19-26. Axial CT, in a different patient, shows fatty dermoid (arrow) in the left anterior nasal cavity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree