Type I (Abernethy malformation): end-to-side communication with the portal system to the IVC (most common), azygous vein, renal vein, right atrium, or iliac veins (Fig. 13.1a)
Type Ia: the splenic vein (SV) and superior mesenteric vein (SMV) each drains separately into the systemic circulation
Type Ib: the SV and SMV join to form a common trunk and directly drain into the systemic circulation bypassing the liver
Type II: side-to-side anastomosis of the portal with the IVC (Fig. 13.1b)
Type IIa: congenital type II shunts
Type IIb: acquired type II shunts
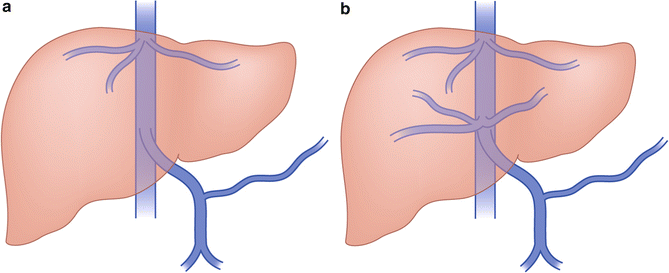
Fig. 13.1
Congenital extrahepatic portosystemic shunts. (a) Type I—end-to-side communication of portal vein to IVC. (b) Type II—side-to-side anastomosis of portal vein with IVC
Table 13.2
Congenital intrahepatic portosystemic shunts (Park classification)
Type I—a single large vessel connecting the right portal vein to the intrahepatic IVC (Fig. 13.2a) |
Type II—one or more communications between the peripheral portal and hepatic vein branches within a single hepatic segment (Fig. 13.2b) |
Type III—type II shunt with an intervening venous aneurysm (Fig. 13.2c) |
Type IV—multiple portosystemic communications between portal and hepatic veins within multiple hepatic segments (Fig. 13.2d) |
Type V—the fifth type of intrahepatic shunt is a patent ductus venosus. This type of shunt is an abnormal persistence of the embryologic vascular connection between the proximal left portal vein and the IVC (Fig. 13.2e) |
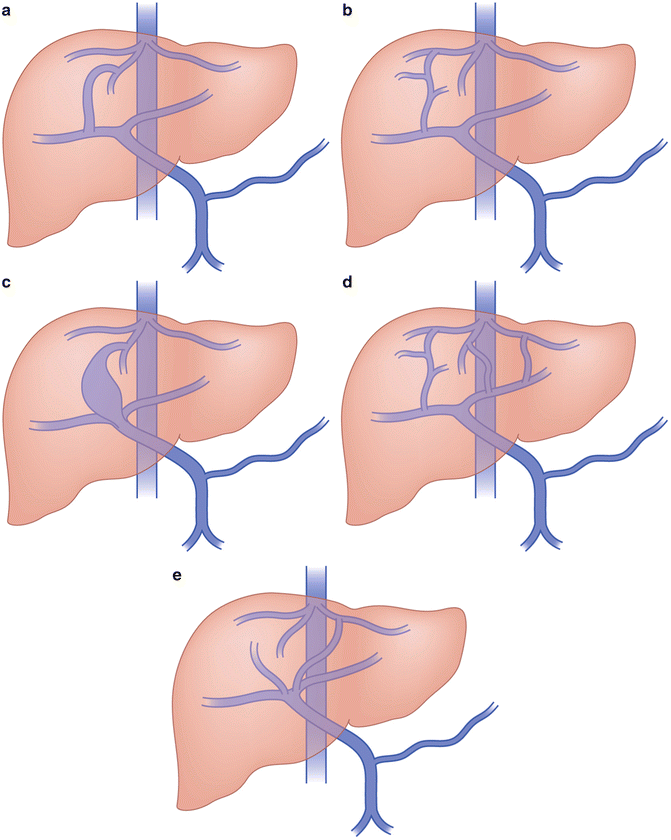
Fig. 13.2
Congenital intrahepatic portosystemic shunts. (a) Type 1—right portal vein to intrahepatic IVC. (b) Type II—peripheral portal to hepatic vein connection, single segment. (c) Type III—type II with aneurysm. (d) Type IV—multiple portal hepatic connections, multiple segments. (e) Type V—patent ductus venosus
Patient Presentation
Regardless of which type of shunt occurs, the presenting symptoms constitute a wide spectrum. CPSS may be asymptomatic or associated with varying degrees of encephalopathy. In fact, the majority of shunts are discovered during screening for metabolic diseases. Due to portal blood bypassing the liver, these patients often have hypergalactosemia, elevated ammonia levels, and sometimes hyperbilirubinemia.
In type I CEPSS, there is a congenital absence of the portal vein with complete diversion of portal blood into the systemic circulation. Intrahepatic portal radicles may be hypoplastic and undetectable [4]. Type 1 shunts are more commonly seen in females and are associated with other anomalies including biliary atresia, polysplenia, malrotation, situs inversus, multicystic dysplastic kidneys, Goldenhar syndrome (oculoauriculovertebral dysplasia), choledochal cyst, skeletal anomalies, and cutaneous hemangiomas.
Type II CEPSS are more common in boys with less association with congenital anomalies but can be seen in patients with pulmonary valve atresia, patent ductus arteriosus, Goldenhar syndrome, polysplenia, and IVC anomalies.
Associated congenital anomalies are much less common with CIPSS but can still occur, e.g., biliary atresia, cutaneous hemangiomas, and congenital heart disease.
Excessive shunting results in accumulation of toxic metabolites such as bile acids, cholylglycine, galactose, and ammonia resulting in varying degrees of hepatic encephalopathy. Signs of portal hypertension such as varices, ascites, and splenomegaly may occur but are infrequent. Features of portal hypertension usually indicate that the existing shunt is compensatory and not congenital. Patients with CIPSS can present with postprandial hyperglycemia seen especially in diabetic patients. Both CEPSS and CIPSS may be associated with the development of intrahepatic lesions (fatty liver, focal nodular hyperplasia, adenoma, nodular regenerative hyperplasia, hepatoblastoma, and hepatocellular carcinoma) which may regress after treatment. Any remaining nodules are generally evaluated with follow-up imaging and do not typically require percutaneous biopsy since the occurrence of hepatocellular carcinoma is rare. It is thought that hepatic tumors occur as a compensatory response to the decrease in intrahepatic flow of blood, but the exact mechanism of hepatic nodule formation is unknown. Hepatopulmonary syndrome (hepatic dysfunction, lung vascular alteration, and hypoxemia) may occur with either type of shunt and can result in significant pulmonary hypertension [5–8].
Preprocedure Workup
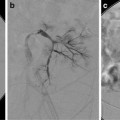
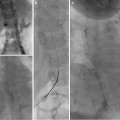
Abdominal ultrasound with hepatic Doppler evaluation is the initial radiologic workup when a malformation is suspected. US evaluation is inexpensive, is noninvasive, and does not expose the patient to radiation. The vast majority of shunts can be visualized with US alone (Fig. 13.3). After the malformation has been recognized, the next step is to utilize cross-sectional imaging (CT or MR) to demonstrate the exact course of the shunt and visualize the remainder of the portal system in order to plan subsequent management. Cross-sectional imaging more accurately demonstrates the extrahepatic venous anatomy, features of portal hypertension (ascites, splenomegaly, varices), and the presence of intrahepatic masses (Fig. 13.4). The advantage of MR imaging is the lack of ionizing radiation. Nuclear medicine studies such as iodine 123 iodoamphetamine administered rectally have been used to calculate shunt ratios between the liver and the lung primarily for type II CEPSS. A shunt ratio of greater than 30 % indicates an increased risk of encephalopathy [9]. Catheter-directed angiography is usually reserved for use during percutaneous intervention. Additional laboratory evaluation such as platelet count and coagulation parameters may also be obtained if a transhepatic intervention is contemplated. Urea and creatinine levels are important to obtain prior to contrast administration. Percutaneous liver biopsy is useful primarily in suspected type I CEPSS to evaluate for hypoplasia or absence of intrahepatic portal radicles.
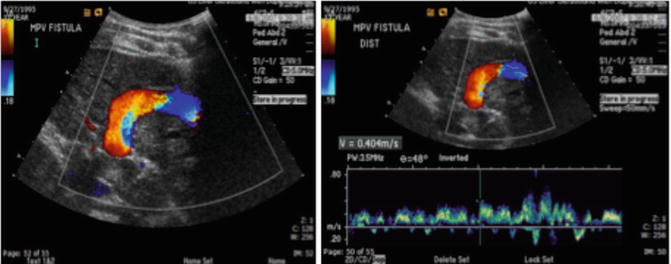
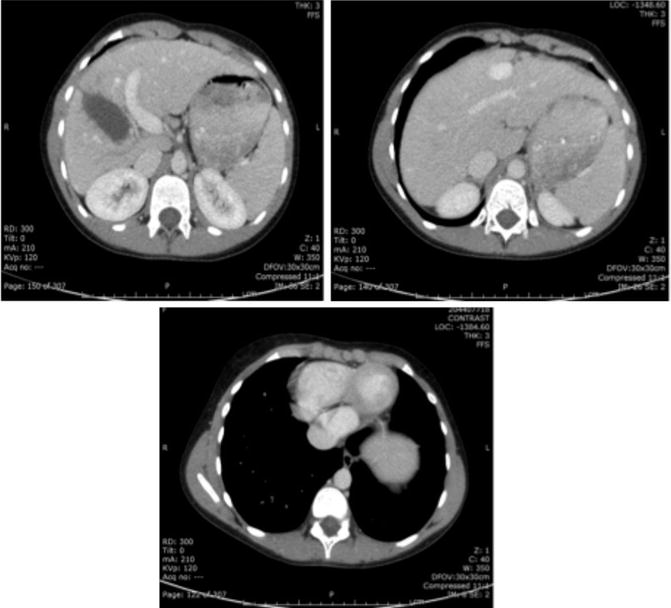

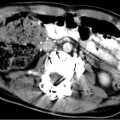
Fig. 13.3
Doppler ultrasound images demonstrating a large congenital shunt from the left portal vein to the IVC (patent ductus venosus)

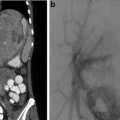
Fig. 13.4
Axial CT images demonstrating a patent ductus venosus. Cross-sectional imaging has the advantage of also illustrating abnormalities within the solid organs
Indications for Treatment
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree