Contraindications, Complications, and Complication Management
The efficacy and safety of ultrasound-guided interventions depend on the type of intervention, the access route, patient-specific risk factors, the experience and expertise of the interventionalist and assistants, respect for contraindications, and the prompt recognition and treatment of complications.
9.1 Interventional Risk
9.1.1 Complication Rates and Mortality
Large retrospective surveys indicate that ultrasound-guided fine needle biopsy (needle diameter up to 1.0 mm) has complication rates in the range of 0.51%1 to 0.81%,2 including a 0.06 to 0.095% incidence of major complications. The mortality rates in these studies range from 0.0011%2 to 0.018%.3 While it should be noted that these studies significantly underestimate complication rates due to their methodologies, they still provide valuable clues to the frequency distribution of biopsy-related complications.
Besides pain, which was not assessed in all the surveys, bleeding is the most common major complication of ultrasound-guided biopsies, followed by infections and malignant seeding along the needle tract. The relative frequency of organ-specific complications (pancreatitis, pneumothorax, bile leakage, abortion) relates to the inclusion of various targeted sites in the statistical data. Deaths were caused mainly by severe hemorrhage, pancreatitis, and sepsis1,2,4,5 as well as needle tract seeding.6 Retrospective and prospective single-center studies with high case numbers that reported on biopsies of the liver and other abdominal organs with needle diameters >1.0 mm have shown markedly higher complication rates from 0.4%7,8 to 2.5%9,10.
9.1.2 Factors that Influence Interventional Risk
Needle Diameter, Needle Type
Data from surveys with high case numbers have yielded controversial results on the diameters and types of biopsy needle used.2,11 Retrospective analyses of parenchymal liver biopsies7,12 and biopsies of focal liver lesions12 have consistently shown higher complication rates associated with the use of cutting biopsy needles compared with aspiration needles. On the other hand, comparative studies do not support the thesis that needle diameters between 18 gauge and 14 gauge (1.2–1.6 mm) are associated with a higher biopsy risk than fine needles.13–18
Number of Biopsies
There is evidence from various studies that the risk of complications depends on the number of biopsies performed.
Target Site
The risk of biopsy depends on the targeted site (see Specific Biopsy Sites in this chapter). Higher complication rates have been reported for the ultrasound-guided biopsy of liver tumors than for parenchymal liver biopsies.8,19
Diagnostic and Therapeutic Interventions
The complication rates associated with ultrasound-guided therapeutic interventions are significantly higher than those in purely diagnostic procedures.8,20,21
Examiner Experience
Data on the relation of complication rates to operator experience are based almost entirely on percutaneous liver biopsies. In a Swiss survey that evaluated 3,501 liver biopsies (only 32.3% of which were ultrasound-guided), the complication rate among internists who performed fewer than 12 biopsies per year (1.68%) was higher than that of physicians who performed at least 50 liver biopsies per year (0%). Gastroenterologists had lower complication rates (0.11%) than internists (0.55%).11 Similar results were reported in a British survey22 and in a retrospective analysis of percutaneous liver and renal biopsies from two U.S. centers.23 A more recent French study that analyzed 600 ultrasound-guided liver biopsies found no significant difference in complication rates between experienced operators (>150 liver biopsies) and inexperienced operators (<15 liver biopsies performed alone). This series included only one major complication, however, and inexperienced operators performed only 25% of the biopsies.24
9.2 Frequent Complications and Their Risk Factors
9.2.1 Pain and Vasovagal Reactions
Pain and vasovagal reactions are frequent complications of ultrasound-guided interventions and occur after 10 to 50% of percutaneous liver biopsies,25 approximately 7% of renal biopsies,26 and approximately 5% of pancreatic biopsies.28 Severe vasovagal reactions were observed in up to 2.8% of patients who underwent prostatic biopsy.28 The pain following liver and splenic biopsies is usually caused by minor bleeding or bile leakage and typically radiates to the shoulders.
9.2.2 Bleeding Complications
Incidence
The largest single-center study published to date was a retrospective review of 15,181 image-guided percutaneous core biopsies (needle diameter at least 20 gauge; kidney, liver, lung, pancreas, others) performed at a British center over a 6-year period.29 Severe bleeding occurred in 0.5% of all cases. The incidence of bleeding was 0.7% after renal biopsy, 0.5% after liver biopsy, 0.2% after lung biopsy, 1.0% after pancreatic biopsy (mostly allografts), and 0.2% after biopsies of other organs (adrenal glands, retroperitoneum, bone, soft tissues, mesentery). This is in good agreement with other data.8,30
Predictors of Bleeding Risk
Several studies have shown that both the platelet count and the prothrombin time (or international normalized ratio, INR) are significant predictors of bleeding risk after percutaneous renal biopsy.8,17,29–31 In the study by Atwell et al, the mean INR was 1.2 ± 0.9 in the small group of patients with severe bleeding but was only 1.0 ± 0.2 in the group without severe bleeding (n = 15,116). The platelet count in the group with major bleeding was 194 ± 88 × 109/L (194 ± 88 × 103/mm3), and thus significantly lower than in the group without bleeding complications (257 ± 111 × 109/L).29 In a multicenter study that investigated the complications of 2,740 percutaneous liver biopsies in patients with hepatitis C and advanced cirrhosis of the liver (HALT-C Trial), bleeding complications (16 cases; 0.6%) were more common in patients with an INR ≥ 1.3, and the rate increased significantly in patients with a platelet count of 60 × 109/L or less (0.6% versus 5.2%). Withholding percutaneous liver biopsy from patients with a platelet count <60 × 109/L would have prevented bleeding in 25% (4 of 16) cases.30 Similar results were found for renal biopsies in an HIV-infected population, where a reduction of platelet count by 10 × 109/L was associated with a 1.05 times higher risk of bleeding.31
In a prospective single-center German study, 8 of 1,923 patients (0.4%) who underwent a diagnostic (n = 1,696) or therapeutic (n = 227) ultrasound-guided intervention of the liver or pancreas suffered bleeding severe enough to require a transfusion. Another 52 patients had a significant fall in Hb (>1.24 mmol/L [2 g/dl]). The bleeding rate was higher in therapeutic interventions (2%) than in diagnostic interventions (liver: 0.12%; pancreas: 1.1%). Severe bleeding did not occur after parenchymal liver biopsy, but the bleeding rate after the biopsy of liver masses was 0.5%. The principal risk factor for bleeding and a fall in Hb in this study8 was hepatic cirrhosis with a Quick value (prothrombin time ratio) <50%, even though fresh plasma infusion was performed in patients with a Quick value in that range (T. Bernatik, personal communication, 2010).
A retrospective analysis of 629 percutaneous liver biopsies at a German center found that 58% of all biopsy-related bleeding occurred in patients with risk factors. In this cohort of patients with a high percentage of patients at risk, the following diseases and factors increased the risk of bleeding events32:
Mycobacteriosis
Need for prophylactic platelet transfusion
Acute liver failure
Heparin administration on the day of the biopsy
Advanced hepatic cirrhosis
Treatment with corticosteroids or metamizole
Hematologic systemic disease
Most reports of fatal bleeding after ultrasound-guided liver biopsies recorded in large surveys involve the biopsy of liver masses (especially hepatocellular carcinoma [HCC] and hemangioma),5,33 although sporadic deaths have also been described in large series of parenchymal liver biopsies. These deaths occurred exclusively in patients with special risk factors (coagulopathy, systemic disease, age).11,22,17,32,34
The biopsy of liver masses not covered by liver parenchyma is often thought to be associated with an increased bleeding risk. While plausible, this hypothesis has not been substantiated by published data.
The following parameters/diseases have been identified as risk factors for bleeding in patients undergoing percutaneous renal biopsy26,35–40:
Arterial hypertension
Acute renal failure
Severe, chronic renal function impairment
Number of needle passes
Female sex
Steroid medication
Increased INR
Hepatitis C infection
Coinfection with HIV and hepatitis C
Amyloidosis
The increased bleeding risk in patients with severe renal function impairment is due mainly to impaired platelet function.41 Data on the role of platelet function tests (traditional bleeding time, PFA-100) for evaluating bleeding risk in renal and liver biopsies are controversial, however.37,42–47
Clinical Significance
The clinical significance of postinterventional bleeding is variable and ranges from asymptomatic hematomas or arteriovenous fistulas detectable by imaging and an asymptomatic drop in serum hemoglobin to symptomatic intraparenchymal, subcapsular, retroperitoneal, pleural, and free intraperitoneal hemorrhages. Rare manifestations are visceral pseudoaneurysms and bleeding into the biliary tract (hemobilia) or urinary tract (hematuria) and bleeding into the pancreatic duct (hemosuccus pancreaticus). There have been isolated case reports of acute cholecystitis, cholangitis, and biliary pancreatitis as a sequel to liver biopsy with bleeding into the biliary system (portobiliary fistula). Severe postinterventional splenic or renal hemorrhage may lead to organ failure.
Infectious Complications and Peritonitis
Antiseptic preparation of the skin site and the use of sterile equipment ensure low rates of infectious complications. On the other hand, abscesses, abdominal or chest wall infections, sepsis, or peritonitis may result from passing a needle or drain through bacterially contaminated spaces (e.g., abscesses, renal pelvis and parenchyma in pyelonephritis, intestinal structures). In a very large, single-center series of various ultrasound-guided procedures (n = 13,534), the rate of postinterventional infectious complications was very low at 0.1%. While no infections were observed after ultrasound-guided thoracentesis or fine needle aspiration, the incidence of infections after ultrasound-guided diagnostic biopsy was 0.2%. Biopsy of a liver transplant had the highest incidence (1%).48 Biliary peritonitis was observed in 0.03 to 0.22% of liver biopsies and resulted from accidental puncture of the gallbladder or passing a needle or drain through bile ducts with obstructed drainage.7,49 Ten septic complications (urosepsis, perirenal abscess; 1%) were found in a retrospective analysis of 1,005 parenchymal renal biopsies, only some of which were ultrasound-guided.35 In another series of 1,090 ultrasound-guided parenchymal renal biopsies, no instances of infectious complications were reported.50
Of course, the risk of infectious complications is particularly high in ultrasound-guided transrectal prostatic biopsies. Uncomplicated urinary tract infections are likely to develop in approximately 11% of cases, fever in 2 to 3.5%, acute prostatitis in 1 to 2%, and urosepsis in only about 0.1 to 0.2%.28,51,52 Risk factors for sepsis after prostatic biopsy are an indwelling transurethral catheter, the presence of diabetes mellitus, and the number of repeat biopsies.53 Isolated cases of fatal septic complications have been reported in the literature. In the event of infectious complications after prostatic biopsy, the causative organisms are likely to be resistant to the antibiotic used for infection prophylaxis (usually a gyrase inhibitor).54 A population-based study in Ontario, Canada, showed that hospital admission rates for urologic complications of transrectal prostate biopsy increased during the years 1996 to 2005, rising from 1 to 4.1%. Most of the hospital admissions (72%) were for infectious complications.55
9.2.3 Needle Tract Seeding
Incidence
Older surveys with large case numbers reported an incidence of 0.003%1 to 0.009%3 for seeding of malignant cells along the needle tract in ultrasound-guided fine needle biopsies of abdominal and retroperitoneal malignancies. One survey reported an incidence of 0.012% after transthoracic needle biopsy.56 It is reasonable to assume, however, that these data understate the true incidence because tumor seeding generally appears with a latency of several months to as much as 25 months after needle biopsy.2,5,57,58 In percutaneous biopsies of hepatocellular carcinomas, needle tract seeding was detected by imaging an average of 267 days (116–619 days) after the procedure.57 There is another factor that contributes to the significant underestimation of needle tract seeding risk in large survey studies: the number of inoculation metastases was related statistically not only to the number of biopsies of malignant masses but to the total number of all ultrasound-guided biopsies.
More recent studies indicate a higher risk of malignant needle tract seeding after both diagnostic and therapeutic ultrasound-guided interventions for malignant tumors.
Liver Metastases
Individual single-center case series showed that tumor seeding after the percutaneous (fine needle) biopsy of colorectal liver metastases occurred with incidences of 10%,6 16%,59 or 19%60 respectively in different series.
Hepatocellular Carcinoma
A literature search published in 2007 yielded 179 cases of tumor seeding after percutaneous biopsy, percutaneous ethanol injection (PEI), or thermal ablation (radiofrequency ablation [RFA] or microwave ablation) of hepatocellular carcinoma (HCC). The median frequency of tumor seeding in the 41 papers reviewed was calculated to be 2.29% after biopsy, 1.4% after biopsy and PEI, 0.61% for RFA, and 0.72% for RFA for biopsy and ablation.61 In another meta-analysis of a total of 8 studies, the incidence of metastatic implants in the needle tract after percutaneous biopsy of HCC was 2.7% (0.9% per year).62 Only one study investigated the possibility of hematogenous tumor cell dissemination but did not demonstrate an increase in circulating tumor cells after the percutaneous biopsy of HCC.63
Lung Cancer and Pleural Mesothelioma
In a series of operatively treated stage I lung cancers, a higher incidence of needle tract seeding and pleural recurrence was found in the subset of tumors that had been confirmed by preoperative percutaneous or intraoperative needle biopsy with an 18-gauge cutting biopsy needle (8.6% versus 0.9%).64 A relatively high risk of needle and drain tract seeding also exists after percutaneous biopsies and interventions for pleural mesothelioma, although the reported incidences vary over a wide range (all interventions: 0–48%; needle biopsy: 0–22%).65 The study with the largest case numbers to date found a 4% incidence of needle tract seeding, which was significantly less than the incidence of tumor seeding after surgical biopsy (thoracoscopy or thoracotomy: 22%).66
Breast Cancer
In a systematic review of a total of 15 studies, epithelial tumor cell seeding after the core needle biopsy of breast cancers was found in an average of 22% of surgical specimens. Preoperative core needle biopsy was not associated with a decrease in survival, however.67
Other Malignant Tumors
Needle tract seeding has also been reported after the ultrasound- or CT-guided biopsy of pancreatic cancers and other malignancies, although no reliable incidence rates are available. There have been individual case reports of pleural, peritoneal, and cutaneous seeding from pancreatic, biliary, and renal cell carcinomas occurring after percutaneous biliary tract drainage and nephrostomy. Weiss et al compiled case data from their own files and from published reports on 29 cases of malignant seeding after ultrasound-guided needle biopsy of dominant pancreatic carcinoma (n = 11) as the targeted site.2 Very few data have been published from single-center studies on the incidence of malignant seeding after the percutaneous biopsy of pancreatic carcinoma. One Japanese group reported an incidence of 1.4% (1 in 73 cases).58
Clinical Significance
In one retrospective comparison, the 4-year survival rates of 90 patients who underwent preoperative biopsy were significantly lower due to a high incidence of needle tract seeding (19%) than in 509 patients who were not biopsied prior to the resection of colorectal hepatic metastases.60 A Spanish study found that the percutaneous biopsy of HCC done before liver transplantation significantly increased the recurrence rate from 5.9% in the group without preoperative biopsy to 31.8% in the biopsy group. The number of hepatic recurrences was roughly the same in both groups (3.9% versus 4.5%). Extrahepatic recurrences (lung, needle tract) developed in 27.3% of the preoperative biopsy group but were rare (2%) in the nonbiopsy group.68,69 Another study in 85 patients with large hepatocellular carcinomas found that preoperative biopsy significantly increased the incidence of postoperative intraperitoneal recurrence (12.5% versus 1.6%) while significantly reducing 5-year disease-free survival (24% versus 52%).70
9.2.4 Specific Complications
Some complications are specific for ultrasound-guided biopsies or interventions in particular organs or lesions, such as pneumothorax and hemothorax after percutaneous chest biopsy, pancreatitis after pancreatic biopsy, hematuria after renal biopsy, hypertensive crisis after biopsy of pheochromocytoma, anaphylactic shock after hydatid cyst biopsy, and injuries to cervical vessels and other structures after biopsies of the thyroid gland, cervical lymph nodes, and salivary glands.
9.3 Prevention of Complications
9.3.1 Risk Assessment and Patient Selection
Selecting patients for ultrasound-guided biopsies and interventions should always be done responsibly while giving due consideration to the expected clinical benefit, the individual procedural risk, the experience of the interventionalist, and alternative techniques. In particular, it should be determined whether the result of a biopsy is likely to influence patient management and whether noninvasive studies (especially contrast-enhanced ultrasonography [CEUS] and other imaging procedures) could provide diagnostic information adequate for treatment planning.
The decision on ultrasound-guided interventions in patients treated with anticoagulants or antiplatelet drugs should be based on a conscientious assessment of procedure-related and thromboembolic risks (▶ Table 9.1, ▶ Table 9.2). The urgency of the intervention should also be considered.
In patients with advanced liver or kidney disease, it should be noted that the increased risk of bleeding complications after percutaneous interventions is based on complex mechanisms and is not fully reflected in global markers such as platelet count, PT (or Quick value), INR, PTT, and bleeding time. The clinical bleeding history is of much greater importance in this regard.71–73 Nevertheless, we still recommend the routine determination of PT or thromboplastin time (Quick value), INR, PTT, and platelet count before any elective intervention, both for legal reasons and for best practice. The determination of bleeding time or a platelet function test with PFA-100 are advised in patients with advanced renal failure or advanced liver damage, and in patients who have a positive bleeding history with normal global coagulation tests.
Caution
The assessment of risks before a percutaneous ultrasound-guided procedure is based mainly on the patient’s history and clinical data.
Global coagulation tests in themselves are inadequate for the assessment of bleeding risk.
9.3.2 Modification of Risk Factors
Interruption of Dual Antiplatelet Therapy
Drawing on current recommendations for endoscopic procedures, aspirin should be stopped 5 to 7 days before an ultrasound-guided intervention and clopidogrel and the new ADP-receptor antagonists should be stopped 7 to 10 days before, if this is considered to be necessary and reasonable (▶ Table 9.1). In patients with a high cardiovascular risk and especially in patients with coronary drug-eluting stents (DES), the procedure and the timing of the intervention should be coordinated with the cardiologist. Ordinarily, clopidogrel and other ADP-receptor antagonists can be discontinued at least 1 month after implantation of a bare metal stent and 12 months after implantation of a DES while aspirin is continued. If an intervention cannot be postponed, only the therapy with clopidogrel and other ADP-receptor antagonists should be interrupted while aspirin is continued.74,75 Aspirin monotherapy does not necessarily have to be stopped.
| High risk | Low risk |
| <12 months after implantation of a coronary drug-eluting stent | Coronary heart disease without a coronary stent |
| <1 month after implantation of a coronary bare metal stent | Cerebrovascular disease |
| <1 month after implantation of a peripheral vascular stent | Peripheral arterial occlusive disease |
Interruption or Reversal of Anticoagulant Therapy
In patients with a low thromboembolism risk (▶ Table 9.2), treatment with traditional oral anticoagulants (vitamin K antagonists, VKAs) can be stopped for approximately 5 to 7 days before the scheduled intervention, and the ultrasound-guided intervention may proceed when the INR is 1.5 or less.76 It should be noted that the anticoagulant effect will subside at varying rates in difference individuals, and this process may take more than one week in patients with an initially high therapeutic INR, impaired liver function, or a low maintenance dose. Novel oral anticoagulants (NOACs, e.g., dabitragan, apixaban, rivaroxaban) in patients with normal renal function should be stopped just 24 hours before a scheduled ultrasound-guided intervention, whereas treatment in patients with renal insufficiency should be interrupted for a longer period depending on the glomerular filtration rate.
| Condition or disease | High risk | Moderate risk | Low risk |
| Mechanical heart valve prosthesis | Mitral valve prosthesis | Bileaflet aortic valve prosthesis plus one of the following criteria:
| Bileaflet aortic valve prosthesis without other risk factors |
| Older aortic valve prosthesis | Biological valve replacement | ||
| Valve replacement and myocardial infarction or TIA ≤6 months | |||
| Atrial fibrillation | Atrial fibrillation and mitral stenosis | CHADS2 score of 3 or 4 | CHADS2 score of 0—2 (and no prior stroke or TIA) |
| Venous thromboembolism | <3 months after VTE | 3–12 months after VTE | >12 months after VTE without other risk factors |
| Severe thrombophilia | Mild thrombophilia (heterozygous factor V Leiden or factor II mutation) | ||
| Active cancer | |||
| Vena cava filter | Recurrent VTE | ||
| Abbreviations: TIA, transient ischemic attack; VTE, venous thromboembolism. Source: reference 76 | |||
In cases where there is an acute or emergent need for an ultrasound-guided intervention, the effects of VKAs can be reversed with PPSB (a prothrombin concentrate: prothrombin = F II, proconvertin = F VII, Stuart-Power factor = F X, antihemophilic factor B = F IX, e.g., Beriplast) or FFP (fresh frozen plasma) or can be antagonized by the oral or intravenous administration of vitamin K. A 5- to 10-mg dose of vitamin K is necessary for the complete reversal of anticoagulation.77 An adequate response to vitamin K use depends on the dose, mode of administration, baseline INR, and liver function and is usually obtained in approximately 24 to 48 hours. The decrease in INR observed only a few hours after the intravenous administration of vitamin K is initially due only to the rise in factor VII concentration; a sufficient rise in factor II concentration takes approximately 24 hours.77 By contrast, PPSB and FFP are active immediately after intravenous administration. PPSB is faster and more effective than FPP for the reversal of anticoagulation and should be preferred for that reason and for its lower volume load and infection risk. One IU of PPSB per kg body weight increases the Quick value by approximately 1%. With a baseline INR of 2 to 3, a PPSB dose of 20 IU/kg BW is usually sufficient to achieve an INR <1.5. Another option is to administer 20 mL of FFP per kg BW.77 The intervention should be performed without delay because the half-life of the administered factors is only 5 to 8 hours and their efficacy will diminish over time. Importantly, the anticoagulant effect of NOACs is not reversible by PPSB or FFP.
Bridging
Bridging with low–molecular weight heparin (LMWH) is indicated in patients with a high or moderately high risk of arterial or venous thromboembolism in cases where VKA therapy will be interrupted or reversed.76 Heparin can be given at a therapeutic dose in patients with a high thromboembolic risk. The therapeutic dose per kg body weight should be reduced in patients with impaired renal function. Registry studies have shown that bridging with a half-therapeutic dose provides adequate protection from thromboembolic events in patients with a moderately high thromboembolic risk.78,79 Prophylactic dosing is adequate in patients with a low thromboembolic risk. The procedure may be scheduled for approximately 6 hours after the last dose of standard heparin or approximately 8 hours after the last dose of a LMWH. In patients with severe renal failure (KDOQI stage 3 or higher), the interval from the last dose of LMWH to the procedure should be lengthened to at least 12 hours. Ordinarily, the last heparin dose before the intervention will be administered on the eve of the procedure.
The continuation of heparin bridging and/or the resumption of oral anticoagulant therapy depends on the course of the intervention. After an uncomplicated biopsy and in the absence of other risk factors for delayed bleeding complications, heparin therapy can be continued the evening after the intervention and oral anticoagulant therapy can be resumed the next day. A longer interval (up to 4 weeks) is recommended after renal biopsy. Decisions should be made on a case-by-case basis following therapeutic interventions and in patients with specific risk factors.
The pharmacologic characteristics of NOACs (short half-life of 8–15 hours) obviate the need for bridging in patients on these drugs.
Caution
Weighing the bleeding risk, cardiovascular risk, and risk of thromboembolism should always be an interdisciplinary, case-by-case decision.
Prevention of Bleeding in Patients with Acquired Hemostatic Disorders
Patients with a low platelet count (<50 × 109/L) or impaired plasmatic coagulation due to liver disease (INR > 1.5; PTT > 50 s) may require the administration of platelet concentrates or FFP, depending on the planned interventional procedure, the target and access route, and the needle diameter. Each unit of platelet concentrate contains approximately 3 to 4 × 1011 platelets and raises the platelet count by approximately 30 × 109/L, although platelet concentrates may be less effective in patients with platelet antibodies, splenomegaly, or sepsis. The necessary dose can be calculated as follows:
 (9.1)
(9.1)
Guidelines on the correction of plasmatic coagulation defects or deficits recommend the transfusion of 10 to 20 mL FFP per kg BW. It should be noted, however, that the effect of PPSB and FFP depends on the pretransfusion INR. For example, in the case of a basic value of INR of 3.0, 2,000 mL of FFP is needed to correct the INR to 1.5. If INR is 5.0, 3,000 mL FFP is necessary.80 Correcting plasmatic coagulation and bleeding risk in patients with mild plasmatic coagulation disorders (INR = 1.1–1.85) is of questionable benefit.80–83 As a rule, the use of FFP is preferred over PPSB in patients with coagulopathy due to liver disease. An (expensive) alternative is recombinant coagulation factor VIIa (NovoSeven, 90–150 μg/kg BW), which can also be used to correct platelet dysfunction.84
A prolonged bleeding time (>10 minutes) in patients with severe renal and liver dysfunction, other types of acquired and congenital platelet dysfunction, von Willebrand disease, mild hemophilia A, or antiplatelet drug therapy can be corrected by the intravenous administration of 1-deamino-8-D-arginine vasopressin (DDAVP, desmopressin). Desmopressin improves primary hemostasis (endothelial platelet adhesion and aggregation) by inducing the endothelial release of large multimers of von Willebrand factor.85–88 It can be administered parenterally (brief intravenous infusion, subcutaneous injection: 0.3 μg/kg BW) or intranasally (≥50 kg BW: 300 μg). A meta-analysis has shown that the prophylactic use of desmopressin in surgical patients can reduce blood loss and lessen the need for transfusion to a slight degree.89 The use of desmopressin to correct a significantly prolonged bleeding time before percutaneous renal biopsy is recommended in several reviews, although there are no hard data to support this practice.47,45,90 Oral conjugated estrogens and dialysis therapy also reduce bleeding time and clinical bleeding events in uremic patients.91
Caution
Preinterventional corrective measures cannot entirely eliminate the increased bleeding risk in patients with acquired hemostatic disorders.
Prevention of Bleeding in Patients with Congenital Hemostatic Disorders
The factor level should be increased to at least 70% in hemophilia patients scheduled for liver biopsy. Recommendations on the duration of the correction range from 1 to 7 days.92 A target level of 50% is considered adequate at some centers (T. Bernatik, personal communication, 2010). An IU factor concentrate will raise the factor level by approximately 2% (factor VIII, hemophilia A) or 1% (factor IX, hemophilia B). A consultation with a hemostasis expert is strongly advised before any intervention.
9.3.3 Risk Reduction Techniques
Prevention of Bleeding
Many methods have been described for making percutaneous biopsies safer in patients with an increased bleeding risk. All employ coaxial techniques to plug the needle tract with a hemostatic agent (Floseal, Gelfoam, Felaspon)93–97 or seal it by radiofrequency cauterization.94,98,99
Prevention of Pneumothorax
The risk of pneumothorax after percutaneous lung biopsy could be reduced in animal experiments and in a small comparative study by plugging the needle tract with fibrin glue.100,101 The use of a coaxial needle with continuous suction also reduced the risk of pneumothorax during CT-guided lung biopsies in experimental animals.102
Prevention of Needle Tract Seeding
Prophylactic radiotherapy to the needle tract is often practiced after the transthoracic biopsy of pleural mesothelioma, although two of three randomized controlled studies showed no benefit from this procedure.65
Further studies are needed to determine whether different methodologies such as the coaxial biopsy technique,103 specific biopsy needles,57 or ablation of the needle tract99 after percutaneous biopsy or other interventions for malignancies can reduce the risk of tumor seeding.
Prevention of Damage to Adjacent Organs during Thermoablation
A thermal protection technique such as the local injection of saline solution (hydrodissection) is an important precaution in cases where the target lesion for thermoablation (radiofrequency ablation [RFA] or microwave ablation [MWA]) is located close to nearby organs (gallbladder, colon) or duct systems (bile duct, urinary tract). The ureter can be splinted in retrograde fashion (see local tumor therapies in Chapter ▶ 19).
9.3.4 Local Anesthesia and Intravenous Sedation
Thorough local anesthesia with a moderately long-acting agent (e.g., lidocaine 1%, prilocaine 1%) is a fundamental requirement for preventing pain, uncontrolled movements, and associated complications during percutaneous interventions. After a skin wheal is raised, the proposed needle tract and especially the peritoneum in the needle path should be adequately anesthetized using a very thin needle (25–22 gauge) under ultrasound guidance. All air should be expelled from the needle and syringe before the injection; otherwise tiny air bubbles would enter the needle track, causing troublesome artifacts that could hamper the biopsy or intervention. A periprostatic nerve block with 1 to 2% lidocaine is effective prior to ultrasound-guided transrectal prostatic biopsy.
Intravenous sedation (midazolam, possibly combined with pethidine, fentanyl, or piritramide) limits the ability of the patient to cooperate with the intervention (respiratory maneuvers) but may be advisable before diagnostic biopsies in very anxious and restless patients. Intravenous sedation is generally necessary for prolonged and painful therapeutic interventions such as percutaneous transhepatic cholangiodrainage (PTCD) and tumor ablation.
Note
Preinterventional patient education and trust are the best remedies in anxious or uncooperative patients.
9.3.5 Prevention of Infection
It is widely agreed that the ultrasound probe used in a percutaneous biopsy should be disinfected, sterilized, or preferably covered with a sterile sheath, especially in therapeutic interventions and in immunocompromised patients. The disinfection and sterilization of ultrasound probes should follow manufacturers’ recommendations, which often prohibit the use of alcohol-based disinfectants (see Chapter ▶ 8). Sterilization is feasible only for biopsy transducers. Alcohol-free wipes (e.g., Mikrobac Tissues from BODE) are excellent for probe disinfection. Following antiseptic skin preparation at the puncture site (e.g., with an isopropyl alcohol–based disinfectant) and sterile draping of the field, a sterile ultrasound gel or disinfectant spray is applied to the skin for acoustic coupling. The operator and any assistants should clean their hands with a hygienic handrub and don sterile gloves. In therapeutic interventions, the intervention team should also wear a sterile gown, mask, and cap, and a sterile instrument table should be prepared104–106 (see Chapter ▶ 8).
Antibiotic prophylaxis (especially with ciprofloxacin and other gyrase inhibitors) is standard practice for ultrasound-guided transrectal prostate biopsy.107–109 One preinterventional dose is sufficient.110,111 Rectal preparation has proven beneficial before ultrasound-guided prostate biopsy.112 Several studies have also shown that a povidone-iodine enema or suppository before transrectal ultrasound-guided biopsy can lower the rate of infectious complications.113–115
9.3.6 Optimal Approach and Alternatives
Numerous studies have shown that liver and renal biopsies performed with ultrasound guidance have lower complication rates than when the biopsy site is selected based on clinical criteria. Thus, imaging guidance (generally by ultrasound) should be used not only for biopsies of focal lesions and therapeutic interventions but also for parenchymal biopsies of the liver and kidney.116–118 One study found that in 15.1% of cases, ultrasound biopsy guidance prompted a change in the biopsy site that had been chosen based only on clinical criteria. Ultrasound guidance facilitated avoidance of intervening blood vessels and other organs.119 Before the needle is introduced, time should be taken to check various patient positions and depths of respiration to find the safest access route to the lesion. Proper selection of the ultrasound probe is also important for a safe and successful intervention. In the case of patients and/or needle paths with a high risk of complications, alternatives to the ultrasound-guided intervention should be considered. In some situations, computed tomography or endosonography will provide safer and easier guidance for lesions that are difficult to visualize with ultrasound or are located in the mediastinum, posterior retroperitoneum, or lesser pelvis. Endosonographic guidance should particularly be considered for the diagnostic biopsy or drainage of lesions located in the posterior mediastinum or close to the gastrointestinal tract, in the left suprarenal area, or in the pancreas. Even very small lesions of the liver and spleen can be biopsied endosonographically with high accuracy and very low risk (see Chapter ▶ 22).120 Other alternatives are a transjugular parenchymal biopsy of the liver or kidney, especially in patients with a high bleeding risk, morbid obesity, or significant ascites121–124; laparoscopic biopsy; and open biopsy.
Note
Invest time and patience in selecting the optimum position and biopsy route (“measure twice and cut once”).
9.4 Contraindications
For the most part, contraindications to ultrasound-guided interventions have not been validated by clinical studies but are based on traditional experience and “good clinical practice.” Of course, an invasive procedure should not be performed if the patient (or guardian) is inadequately informed or has not consented to the procedure (▶ Table 9.3). This principle may be violated only in life-threatening situations and emergent procedures to which the patient cannot give informed consent. Inability of the patient to cooperate with the procedure or inadequate sonographic needle guidance may create a situation in which an ultrasound-guided procedure cannot be performed with reasonable risk or must be terminated.
| Type (absolute or relative contraindication) | Contraindication |
| Absolute contraindications | Lack of patient education and informed consent |
| Lack of patient cooperation | |
| Biopsy result is unlikely to influence further diagnostic and therapeutic actions | |
| Poor ultrasound needle guidance | |
| Treatment with oral anticoagulants or severe plasmatic coagulation disorder: INR >1.5, PTT >50 s, platelets <50 × 109/L, therapeutic heparinization, treatment with thrombin inhibitors (e.g., hirudin, dagibatran) or factor Xa antagonists (e.g., fondaparinux, rivaroxaban, apixaban, edoxaban) | |
| Treatment with antiplatelet drugs of the thienopyridine class or ADP-receptor antagonists (clopidogrel, ticlopidine, prasugrel, ticagrelor) or glycoprotein IIb/IIIa receptor antagonists (abciximab, eptifabatide, tirofiban) | |
| Relative contraindications | Preoperative biopsy of resectable liver malignancies |
| High-risk access route (blood vessels, bowel) | |
| Target lesion assumed to have a high bleeding risk (e.g., suspected hepatic adenoma or subcapsular hemangioma) | |
| Superficial lesions in parenchymal organs (scant parenchymal coverage) | |
| Close proximity of the target lesion for ultrasound-guided ablation to major vessels, the gallbladder, or tubular structures (bile duct, renal pelvis, ureter) | |
| Adrenal masses whose differential diagnosis includes pheochromocytoma | |
| Suspected hydatid cyst |
Before every biopsy, it should be determined by interdisciplinary consultation whether the result is likely to have a significant impact on further diagnosis and management. Since there is a relative risk of seeding tumor cells along the needle tract, adversely affecting the prognosis, preoperative biopsy confirmation should generally be withheld in patients with presumed liver malignancies that are considered resectable.125
9.4.1 Coagulopathies
The limits stated in textbooks and reviews for parameters such as platelet counts and plasmatic coagulation factors differ slightly from one another, due mainly to a lack of confirmation by controlled studies. The figures stated in ▶ Table 9.3 should be considered as guidelines only. They may have to be modified in selected cases depending on the urgency of the indication, the risk of the proposed intervention, and individual risk factors for bleeding complications. In one series of 47 patients with focal liver lesions and severe coagulopathy (platelet count <50 × 109/L and/or prothrombin time ratio <50%) who underwent fine needle liver biopsy guided by color Doppler ultrasound, there were no instances of severe bleeding, and minor complications occurred in only 3 patients (8.5%).126 Embolization of the needle tract with procoagulant agents after biopsy was found to be a safe technique in similar populations.96,127,128 Any departures from the above contraindications should be noted during the informed consent process and should be documented along with the reason for deviating from standard recommendations.
9.4.2 Procoagulant Therapy and Antiplatelet Drugs
Regarding treatment with procoagulant agents and antiplatelet drugs, it is important to weigh the benefits and risks of an ultrasound-guided intervention on the one hand and the discontinuation or reversal of this therapy on the other hand, as well as the urgency of the proposed intervention. In making this decision, the indication for anticoagulant or antiplatelet therapy must be known. In some cases the risks should be assessed in consultation with the prescribing physician (cardiologist, angiologist, vascular surgeon, neurologist). The risk assessment should be expressly noted during informed consent and should be documented. Pharmacologic parameters should be considered in timing the intervention and in reversing anticoagulant or antiplatelet therapy.
9.4.3 “Risky” Lesions and Access Routes
Hemangiomas, among others, have been cited in the literature as “risky lesions” for ultrasound-guided biopsy. Nevertheless, small case series have been published that report the uncomplicated ultrasound-guided biopsy of liver hemangiomas.129,130 There is even more evidence to document the safety of ultrasound-guided biopsies of splenic parenchyma and focal splenic lesions.13,131–136
The access route for a needle biopsy is considered a relative risk that depends on the experience of the interventionalist. The ultrasound-guided percutaneous biopsy of deep retroperitoneal lymph nodes as well as mesenteric, gastrointestinal, perivascular, and splenic masses was long considered to be a risky procedure. But ultrasound guidance, with its capability for real-time visualization of target lesions in various planes and for graded compression, is useful for optimizing the access route. Indeed, studies in recent years have shown that even the lesions mentioned above as involving “risky access” can be biopsied under ultrasound guidance with little risk106,137–147 (see Section ▶ 9.6).
Caution
When relative contraindications are present, it is important to weigh the risks carefully and also to make a critical appraisal of one’s own expertise.
9.5 Management of Complications
9.5.1 Postinterventional Care and Detection of Complications
Ultrasound imaging is performed immediately after the intervention to check for new fluid collections, free air, pneumothorax, catheter malposition, or other evidence of a complication. A sign suggestive of clinically significant bleeding is the persistence of a “patent track,” or flow along the needle tract detectable by color duplex scanning (▶ Fig. 9.1).
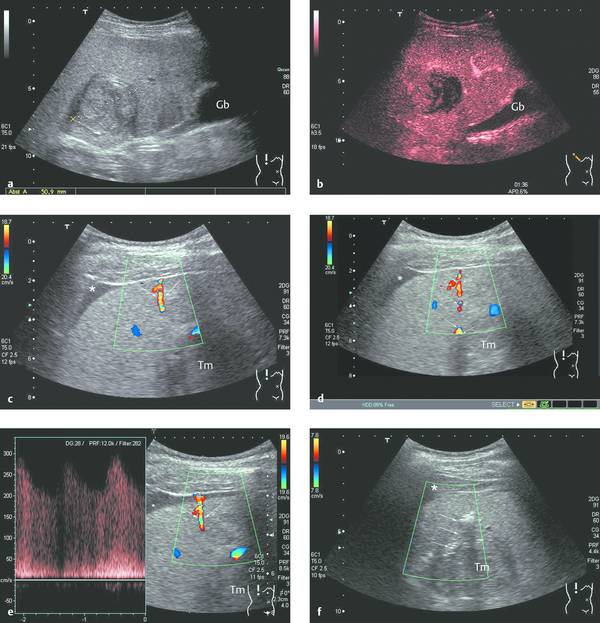
Fig. 9.1 “Patent track sign” and intra-abdominal bleeding after biopsy (18-gauge BioPince, Pflugbeil) of a liver mass in a cirrhosis patient (male, 60 years old; histology: hepatocellular carcinoma in a setting of hemochromatosis).
a Heterogeneous mass approximately 5 cm in diameter with an echogenic pseudocapsule (Gb: gallbladder).
b On contrast-enhanced ultrasound (1.5 mL SonoVue, Bracco) the mass shows arterial-phase hypervascularity with partial washout in the late phase.
c, d Image at 1 min post biopsy (18-gauge BioPince) shows a hypoechoic stripe between the liver and peritoneum (asterisk) and a high-frequency flow signal (arrows) between the tumor mass (Tm) and liver capsule. Blood flow past the liver capsule is also well defined by PW Doppler.
e, f When bleeding persisted for more than 5 min and the patient developed pain and hypotension, thrombin solution was injected into the biopsy tract with a 22-gauge needle under sonographic guidance. Bleeding ceased immediately after the injection (arrows: high-level echoes after thrombin injection). The initially hypoechoic subphrenic hematoma appears markedly more echogenic 25 minutes after the onset of bleeding (asterisk). It was no longer detectable the following day.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree