Contrast Agent Safety and Nephrogenic Systemic Fibrosis
Moazzem Kazi
Georg Bongartz
Martin R. Prince
Nephrogenic Systemic Fibrosis
Introduction
Nephrogenic systemic fibrosis (NSF) is a rare disease typically found among individuals with severe renal impairment. The first cases thought to be NSF occurred as early as 1997 in renal dialysis patients having a “scleromyxedema-like cutaneous disease.”1 Because early reports of NSF had multiple dermatologic findings, the condition was initially termed “nephrogenic fibrosing dermopathy.”2,3,4,5 It soon became evident, however, that this emerging disease entity could affect multiple organ systems in addition to the skin, such as the heart, lungs, liver, and muscles, so the more descriptive term “nephrogenic systemic fibrosis” was adopted.
Epidemiology
NSF affects men and women equally and cases have been reported in Caucasian, African, Asian, Indian, and Hispanic individuals. Most cases have been identified in the United States, followed by Europe and Asia.6 The lower incidence in Asians is hypothesized to be more likely because of the lower gadolinium contrast doses used in Asia rather than any racial differences. There is a strong connection between NSF and poor renal function. Grobner’s landmark observation connecting renal function, gadolinium contrast, and NSF found that five out of nine hemodialysis patients developed NSF after the administration of high-dose nonionic linear gadolinium contrast.
TABLE 8.1 PROPERTIES of GADOLINIUM COMPOUNDS COMMERCIALLY AVAILABLE | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Nephrogenic Systemic Fibrosis Risk Factors
Renal Function
One study looking at NSF incidence among patients receiving gadolinium contrast at two large medical centers in the United States7 found that all patients developing NSF had an estimated glomerular filtration rate (GFR) less than 30 mL/min/1.73 m2. No patient developed NSF after receiving standard-dose gadolinium contrast. But 0.17% (n = 8997) of patients developed NSF after receiving high-dose gadolinium contrast. The incidence of NSF associated with high-dose gadolinium increased from 0.4% among patients getting chronic hemodialysis to 8.8% among patients with a GFR less than 15 mL/min/1.73 m2 but who were not on hemodialysis. The study concluded that acute renal failure and delayed hemodialysis were NSF risk factors for patients with a GFR less than 30 mL/min/1.73 m2 who received high-dose gadolinium.
Gadolinium Agent Administered
In addition to renal function, the type (structure) and dose of gadolinium contrast administered are other possible risk factors for NSF.8 Gadolinium contrast agents can be classified as having either macrocyclic or linear structures (see Chapter 7). The structures can be further subdivided into ionic and nonionic compounds. These different structures of gadolinium chelates have different chemical properties inside the body, such as thermodynamic and kinetic stability, which reflect how likely and how quickly gadolinium can be released by the chelator (Table 8.1). Macrocyclic gadolinium complexes
(e.g., gadoteridol) are usually more stable than ionic linear complexes (e.g., gadobenate dimeglumine), which are in turn more stable than nonionic linear complexes (e.g., gadodiamide). Once in its free form, gadolinium ions (Gd3+) may recombine with the chelator or form complexes with phosphate or other anions, interfere with enzymatic action, or interfere with transmembrane channel function. Gadolinium–phosphate complexes may precipitate and deposit in tissues. Cations, such as Zn+2, Mg+2, and Ca+2, may accelerate the release of gadolinium ions from the chelator in a process called “transmetallation.”9
(e.g., gadoteridol) are usually more stable than ionic linear complexes (e.g., gadobenate dimeglumine), which are in turn more stable than nonionic linear complexes (e.g., gadodiamide). Once in its free form, gadolinium ions (Gd3+) may recombine with the chelator or form complexes with phosphate or other anions, interfere with enzymatic action, or interfere with transmembrane channel function. Gadolinium–phosphate complexes may precipitate and deposit in tissues. Cations, such as Zn+2, Mg+2, and Ca+2, may accelerate the release of gadolinium ions from the chelator in a process called “transmetallation.”9
Gadolinium Dosage and Blood Properties
Higher doses of gadolinium contrast appear to be a risk factor for NSF when compared to the standard dose of 0.1 mmol/kg.8 Acidosis has been suggested as a risk factor for NSF. Extra protons in acidic blood are hypothesized to compete with gadolinium for binding sites on the chelator, reducing chelator stability. Hyperphosphatemia also appears to be a risk factor for NSF. One hypothesized mechanism is phosphate binding to free gadolinium, which precludes recombining with the chelator. The phosphate–gadolinium complex can form a precipitate and decrease the excretion of gadolinium from the body. This also correlates with chronic renal failure being a risk factor for NSF, because elevated blood phosphate is commonly found in chronic renal failure. Proinflammatory events are also associated with increased risk of NSF. These may include recent surgery, acute thrombosis, myocardial infarction, sepsis, and other inflammatory conditions that increase the number of circulating fibrocytes.10 The circulating fibrocyte is hypothesized to be the critical cell that is inadvertently stimulated to produce aberrant fibrosis in NSF.
Signs and Symptoms
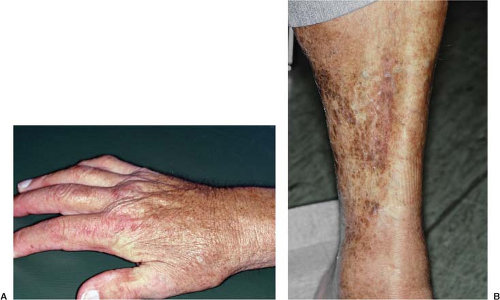
Skin involvement is usually the first symptom of NSF (Table 8.2).11 Patients typically complain of pain, pruritus, tightness, and/or swelling of the skin in the extremities. Lesions appear to be symmetric and bilateral and also tend to be hyperpigmented or erythematous. Hypopigmented lesions are rare. NSF lesions are most frequent in the lower extremities, followed by the upper extremities and then the trunk (Fig. 8.1). The lesions can be as mild as plaques and papules limited to the superficial layers of the skin. NSF lesions may be deeper in the subcutaneous layers, however, where fibrosis can tether skin to the underlying fascia (Fig. 8.2). This may create patterns of skin dimpling or cobblestoning. Joint stiffness and contractures may interfere with normal mobility, and internal organ involvement has been found in autopsies.
TABLE 8.2 SIGNS AND SYMPTOMS, PHYSICAL EXAMINATION, AND LABORATORY TESTS FOR NEPHROGENIC SYSTEMIC FIBROSIS (NSF) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||
Indurated plaques of the deep subcutaneous layers of the skin are often described as firm, thickened, “lumpy,” and shiny (Fig. 8.3).11 A peau d’orange (“peel of orange”) appearance may result when there is extensive dermal involvement. Cobblestoning is commonly found in the thighs and banding is commonly found in the arms or legs.
Physical Examination
A full-body skin examination should be performed in patients with suspected NSF by a dermatologist, rheumatologist, or other health care professional who is familiar with clinical features of NSF and how to distinguish them from other disorders that are in the differential diagnosis of NSF.12 During the skin exam, lesions should be assessed to find ones that are the most suitable candidates for deep dermal biopsy. An examination of the joints should include assessment for loss of range of motion and flexion contractures (Fig. 8.4). An eye exam should also be performed to check for scleral plaques.
Laboratory Tests
Determining the estimated GFR as an indicator of kidney dysfunction is essential in determining who is at risk for NSF. This is most commonly done by measuring a patient’s serum creatinine and incorporating it into the Modification of Diet in Renal Disease (MDRD) equation to calculate GFR.13,14 This calculation also incorporates factors like a patient’s age, gender, and ethnicity when calculating the GFR. To increase the accuracy of the GFR calculation, serum creatinine needs to be collected during steady-state conditions (i.e., outside the setting of an acute kidney injury, perioperative period, etc.).
Differential Diagnosis
There are multiple other conditions with prominent dermatologic findings that are in the differential diagnosis of NSF. Among these are lipodermatosclerosis, scleroderma, scleromyxedema, and eosinophilic fasciitis.11 Lipodermatosclerosis can be distinguished from NSF because lipodermatosclerosis is characterized by painful induration, which usually results from venous insufficiency and venous
stasis. Scleroderma is differentiated from NSF based on physical examination findings of a tapering of the fingertips (i.e., sclerodactyly) and serum autoantibodies SCL-70 and antinuclear antibody (ANA) in scleroderma. Scleromyxedema is distinguished from NSF because scleromyxedema usually involves fibrosis in the upper extremities and face, which are rarely affected by NSF. Although eosinophilic fasciitis may share some fibrosing patterns of NSF, peripheral eosinophilia is more commonly found in the blood of patients with eosinophilic fasciitis.
stasis. Scleroderma is differentiated from NSF based on physical examination findings of a tapering of the fingertips (i.e., sclerodactyly) and serum autoantibodies SCL-70 and antinuclear antibody (ANA) in scleroderma. Scleromyxedema is distinguished from NSF because scleromyxedema usually involves fibrosis in the upper extremities and face, which are rarely affected by NSF. Although eosinophilic fasciitis may share some fibrosing patterns of NSF, peripheral eosinophilia is more commonly found in the blood of patients with eosinophilic fasciitis.
Histopathology
The histopathologic hallmark of NSF is excessive dermal fibrosis.11 Fibrosis is caused by an increased number of fibrocytes in the dermis (Fig. 8.5). Fibrosis can be in focal areas or in the entirety of the dermis, with sparing of the epidermis. Fibrocytes show indistinct cytoplasmic borders on hematoxylin and eosin (H & E) stain. They lack mitotic figures and nuclear membrane abnormalities. In addition, granular material can be found within fibrocyte cytoplasm, which can be detected with Perls’ stain. Fibrocytes are also CD34 positive and procollagen I positive on immunohistochemical staining. Under normal conditions, fibrocytes, which are usually found in bone marrow, begin to circulate and deposit in areas that need wound repair. Through the use of specialized microscopic techniques and spectroscopy, gadolinium has been detected in the affected tissues of NSF patients. The deposition of gadolinium is hypothesized to trigger circulating
fibrocytes to produce inflammation and collagen inappropriately. Deep incisional biopsies sometimes demonstrate the extension of the fibrotic processes beyond the dermis through the underlying fascia, all the way to skeletal muscle.
fibrocytes to produce inflammation and collagen inappropriately. Deep incisional biopsies sometimes demonstrate the extension of the fibrotic processes beyond the dermis through the underlying fascia, all the way to skeletal muscle.
Other histopathologic features of NSF include the absence of both microthrombi and vasculitis, despite being associated with a hypercoagulable state.11 Neovascularization can be present at times and histiocytes are commonly seen. There are usually no excess neutrophils or eosinophils seen, and no inflammatory response occurs in NSF.
TABLE 8.3 FEATURES OF NEPHROGENIC SYSTEMIC FIBROSIS (NSF) ON IMAGING15 | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree