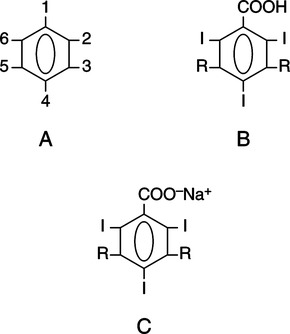
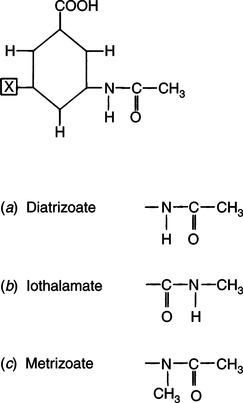
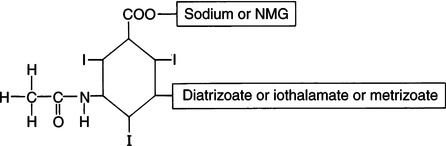
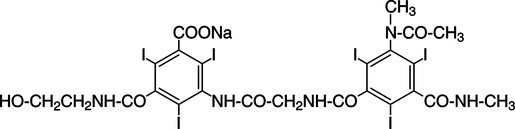
CHAPTER 6 After completing this chapter, the reader will be able to perform the following: Unlike the pharmaceuticals discussed in Chapter 5, the amounts used are much greater than those used for therapeutic drugs. They are designed to remain in the organ of interest for a very short period of time and are not designed to cause any changes either biologically or chemically in the body. Osmolarity and osmolality were discussed in Chapter 5. The blood-brain barrier separates the parenchyma (organ tissue) of the central nervous system from the blood, preventing or slowing the passage of potentially harmful substances from the blood into the tissue of the central nervous system. The hyperosmolality of the ionic organic iodine contrast agents is at the root of many of the major reactions that occur during radiographic procedures. One example of such a reaction is the degradation of the ionic contrast agents into electrically charged particles that can potentially disrupt the heart’s electrical activity, thereby increasing the risks for arrhythmia. This would lead one to think that it would be advantageous to use nonionic contrast agents exclusively in radiography. Adopting a policy of this nature would be easy from a medical standpoint, but there are economic considerations that make the decision difficult. The cost of the nonionic contrast agents is somewhat greater than that of ionic agents. At the present time, the ionic contrast agents are still used; however, the nonionic or low osmolality contrast media have become the primary type of agent used in the diagnostic and interventional arena. Most institutions screen their patients for a variety of risk factors, and based on this information the radiologist will select the dose and type of contrast media to be used. Depending upon the results of the screening process, it is possible to reduce the use of the low osmolality contrast media. In some institutions the use of nonionic contrast media is restricted to high-risk patients or individuals in which the history identifies a high potential for reaction. This type of protocol can result in a substantial economic saving without a corresponding increase in moderate or severe reactions. Almost all of the institutions performing angiographic studies are using the nonionic contrast agents exclusively despite the drawbacks of cost and lack of adequate reimbursement. The American College of Radiology has developed guidelines for the use of the low osmolality and nonionic contrast media.1 The ionic contrast agents used for vascular studies are salts of organic iodine compounds such as benzene. The basic configuration can be drawn as a six-sided ring structure (Fig. 6-1, A). The locations marked 1 through 6 on the illustration represent places where chemical structures can be attached. Figure 6-1, B, shows the placement of the iodine atoms on the molecule. The addition of iodine atoms in the 2, 4, and 6 positions and the conversion of the acid molecule (COOH) to a salt (Fig. 6-1, C) are the basis for the organic iodine compounds used today. The locations marked with the letter “R” are the sites used to place various side chains. The side chains initially were amino groups that helped to decrease the toxicity of the compound. The anions of the common angiographic contrast media begin as organic benzoic acid compounds. The different angiographic contrast agents have the same basic ring structure but can differ in the side chains located at the 3 and 5 position (Fig. 6-2). Different side chains are used to improve solubility and tolerance to the material. The anions are combined with a cation to form a salt (Fig. 6-3). The major cations used to form the compound can be either sodium or meglumine (n-methylglucamine). Each of these salts has different characteristics and will affect the patient differently. These substances were developed because the high osmolality of the ionic contrast agents was thought to be responsible for the majority of the undesirable side effects. The basic structure of the nonionic compounds is the same as that of the conventional agents. However, the various side chains added to the basic building block create a substance that does not disassociate (i.e., separate into an anion and a cation) (Fig. 6-4). They are considered to be 3:1 compounds—they contribute three iodine particles in solution to provide contrast and only one particle to provide osmolality. The ionic compounds are 3:2 in that they also contribute three iodine particles for contrast and two particles for osmolality. This type of contrast agent overlaps characteristics of the two previously mentioned types of substances; the compound uses the same basic building block but forms what is called a monoacid dimer. This compound is an ionic contrast agent, but it has the same type of compound ratio as the nonionic substances. The contrast agent illustrated in Figure 6-5 is a 6:2 compound; that is, it provides six iodine particles for contrast and two particles for osmolality. This type of contrast agent lies between ionic and nonionic agents in the mediation of side effects. Its advantages are that it more closely mimics the nonionic compounds and is not as costly. In comparing contrast agents, the amount of iodine delivered per second to the patient should be considered because contrast agents with a greater amount of meglumine salt usually have a lower iodine content as a result of the increased weight of the meglumine ion. Therefore, the amount of iodine delivered per second will be smaller. To illustrate this, if we compare the iodine content of Hypaque (sodium 50%) with that of both Conray (meglumine 60%) and Renografin 60% (meglumine 52%, sodium 8%) (Table 6-1), it can be seen that the Hypaque has a greater iodine content. It should be noted that Hypaque 50% contains 100% sodium salt, whereas Renografin 60% is a mixture of sodium and meglumine salts, and Conray 60% contains 100% meglumine salt. Table 6-2 illustrates the characteristics of some of the low osmolality and nonionic contrast agents. TABLE 6-1 Characteristics of Some Ionic Intravascular Contrast Media
Contrast Media
 Identify the necessity for the use of contrast agents
Identify the necessity for the use of contrast agents
 List the types of contrast agents that are currently available
List the types of contrast agents that are currently available
 Describe the classes of contrast agents
Describe the classes of contrast agents
 Describe the evolution of the organic iodine contrast media
Describe the evolution of the organic iodine contrast media
 Define the characteristics of a good contrast agent
Define the characteristics of a good contrast agent
 Describe the excretory pathways of the various contrast agents
Describe the excretory pathways of the various contrast agents
 Define informed consent as it applies to contrast media studies
Define informed consent as it applies to contrast media studies
 List and describe the general precautions that should be applied to every contrast medium study
List and describe the general precautions that should be applied to every contrast medium study
 Identify the reactions and complications resulting from the use of contrast agents
Identify the reactions and complications resulting from the use of contrast agents
CLASSES OF RADIOPAQUE CONTRAST MEDIA
Evolution of the Organic Iodine Class of Contrast Media
Ionic Organic Iodine Compounds
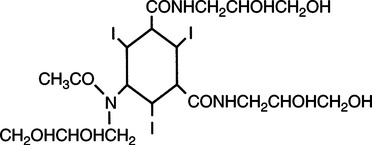
Nonionic Contrast Agents
Low Osmolality Ionic Contrast Agents
Characteristics of Radiopaque Contrast Media
Iodine Concentration
Iodine Content
Chemical Components
Viscosity (cP)
Sodium Content
Agent
Iodine Concentration %
mg/ml
%
Anion
Cation*
25° C
37.5° C
mEq/ml
mg/ml
Hypaque 60%
28 – 31%
282
28
Diatrizoate
Mg (60%)
6.22
4.18
0.001
0.02
Conray 60%
282
28
Iothalamate
Mg (60)%
6.10
4.0
0.0014
0.03
Renografin 60
288
29
Diatrizoate
Mg (52%)
6.0
3.9
0.16
3.76
Hypaque 50%
300
30
Diatrizoate
Na (8%)
3.25
2.5
0.8
18.1
Na (50%)
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access