
Coronary Vein Embolization
Major hemorrhage from bleeding esophageal varices is one of the most challenging clinical entities that the gastroenterologist, surgeon, and interventionalist will face. Because variceal hemorrhage is a major factor in more than 50% of the deaths in the cirrhotic population, the need for developing an effective means of treatment has resulted in a multidisciplinary approach requiring coordinated care from a number of different specialists.
Acute care for patients with bleeding gastroesophageal varices usually involves fluid replacement and blood transfusions to prevent hypovolemic shock and intravenous Pitressen for variceal vasoconstriction. This regimen can control bleeding in up to 56% of patients.1 More recently, propranolol has been used to decrease portal venous pressure. In patients who continue to bleed, esophageal and gastric balloon tamponade can be used to control bleeding in 56 to 92% of patients, but the complication rate with this method of therapy is often significant.2–4 Emergency surgical shunting procedures are generally avoided because of the overwhelming mortality rate. Orloff et al5 demonstrated an operative (30-day) mortality rate of 42% and a 34% rate of protein-related encephalopathy in patients undergoing emergency portocaval shunting for persistent esophageal hemorrhage.
Lunderquist and Vang6 first described percutaneous transhepatic embolization (PTE) of the left coronary vein (LCV) in patients with portal hypertension in 1974. Since its inception, varying reports on its effectiveness and its complication rate have resulted in sinusoidal levels of interest. Despite the introduction of and enthusiasm for endoscopic methods of variceal banding and sclerotherapy, however, a rebleed rate of 28 to 58% has shown that these techniques cannot be used as the sole modality for treatment.7,8 Coronary vein embolization (CVE) remains a valuable option when noninvasive medical and endoscopic options have been used and variceal hemorrhage cannot be controlled or recurs. In contrast to the original transhepatic approach, however, CVE procedures are performed primarily as an adjunctive procedure when transjugular intrahepatic portosystemic shunting fails to result in effective variceal decompression. Although access to the portal venous system is obviously quite different when the transjugular transhepatic approach is used compared with the standard right midaxillary line transhepatic approach, the technique for embolization of the varices remains similar.
This chapter discusses the techniques, embolic agents, complications, and short- and long-term results associated with percutaneous transhepatic and alternative methods of left CVE (LCVE). (See Chapters 4 and 5 for additional information on TIPS-related CVE.)
 Patient Preparation and Contraindications
Patient Preparation and Contraindications
As a result of poor nutritional status, decreased synthetic liver function, anemia, and thrombocytopenia secondary to splenomegaly and hypersplenism, the coagulation status in the cirrhotic patient is often grossly impaired. All patients scheduled to undergo CVE should have a prothrombin and partial thromboplastin time ratio less than or equal to 1.3 × control and a platelet count greater than 50,000/μL. Appropriate transfusions of whole blood, platelets, or plasma should be administered and all hematologic studies rechecked before initiation of the procedure. Renal function also can be impaired; thus, knowledge of the blood urea nitrogen (BUN) and creatinine levels is important because patients may receive a considerable volume of contrast during the procedure.
Ascites is a relative contraindication to PTE of the LCV is performed if the percutaneous transhepatic approach is to be used. The increased distance placed between the liver and right lateral chest wall and accentuated liver motion during respiration can significantly impair catheter control and can result in inadvertent loss of access. Paracentesis should be performed in all patients with a significant amount of ascites before PTE is done unless a transjugular or umbilical vein approach is planned. Broad-spectrum antibiotics that cover both gram-negative and gram-positive organisms also should be administered.
Patients should be fully monitored during the entire procedure. A narcotic such as fentanyl (25–50 μg aliquots) and anxiolytic such as midazolam (0.5–1.0 mg aliquots) can be used concurrendy for effective pain control and anxiolysis. With the use of these agents, two important factors must be kept in mind: (1) These agents work synergistically and can depress respiration markedly; therefore, they can be used only in patients who are fully monitored. (2) Both agents are metabolized by the liver; thus, their effects can be prolonged and accentuated in patients with impaired liver function. Therefore, if these agents are used for pain control and anxiolysis, they must be carefully titrated for each patient under the most controlled conditions.
 Technique
Technique
Access to the portal vein for LCVE has been described as being from the transhepatic,9–11 transjugular vein,12 mesenteric vein (minilaparotomy),13 and umbilical vein14,15 approaches. Before undergoing a LCVE procedure, all patients should undergo celiac angiography to rule out an alternative source of hemorrhage, such as a Mallory-Weiss tear, gastric or duodenal ulcer, portal gas-tropathy, or gastritis. The portal venous phase should be evaluated for patency of the splenoportal axis. A superior mesenteric artery arteriogram also should be performed to clear the arterial side as the source of hemorrhage and to document patency of the mesoportal axis; these steps would be important if the superior mesenteric vein to portal vein approach is to be used. Endoscopy also may be used to confirm that the source of hemorrhage is, in fact, variceal.
Percutaneous Transhepatic Approach
The percutaneous transhepatic approach is the most familiar technique to most radiologists because the procedure for obtaining portal vein access is analogous to that used during percutaneous transhepatic cholangiography and percutaneous biliary drainage. Even early studies performed before the development of the newer hydrophilic guidewires and catheters yielded a procedural success rate of up to 94%.11,16,17 To perform the procedure, the patient is placed in a supine position, and fluoroscopy is used to note the most inferior position of the right lung on deep inspiration. The intercostal space inferior to the lung’s position on inspiration (usually 9–10 or 10–11) is infiltrated with 10 mL of 1% Xylocaine at the midaxillary level. A no. 11 scalpel blade is used to make a small stab wound, which is subsequently spread with Kelly clamps or a hemostat. A 15–cm 22-gauge Chiba needle is inserted parallel to the table and posterior ribs and under fluoroscopic guidance is advanced to the midline. The patient is asked to suspend respiration while the needle is inserted. The stylet is removed, and the needle is connected to a syringe of contrast by a connecting tube. The needle is slowly withdrawn as gentle puffs of contrast are injected. When the needle crosses the portal vein, the characteristic branching pattern is noted. An alternative technique is to withdraw the needle slowly under negative pressure without fluoroscopy. When blood is aspirated through the needle, contrast is injected gently. The procedure is repeated until a portal vein branch is located. If either technique fails to locate a portal vein branch on the first pass, a grid or fan-type pattern is used in which five to seven subsequent needle passes are performed 1 cm apart (through the same transhepatic tract). This pattern is repeated 1 cm above and below the original plane until a portal vein radicle is entered. When a portal vein branch is located, a 0.018– inch Cope mandrel wire (Jeffrey Wire Guide Exchange Set, Cook, Bloomington, IN) is passed through the needle and into the portal vein. The 22-guage needle is removed, and the Jeffrey sheath-dilator-assembly is passed over the guidewire until it reaches the portal vein. The stiffening cannula is unscrewed from the sheath-dilator unit, and the dilator and sheath are advanced together over the guidewire into the portal vein. The dilator is removed and a hemostatic valve (Cordis Corp, Miami, FL) is attached to the sheath.
Alternately, a 0.35-inch 3-mm J Rosen (Cook, Bloomington, IN) guidewire can be passed through the Jeffrey’s sheath into the portal and superior mesenteric veins. The Jeffrey’s sheath is then exchanged for a 6F vascular sheath (Terumo Corporation, Medi-Tech, Watertown, MA), which is attached to a low-volume power flush and stitched in place.
Once the sheath is in place, a 5F pigtail catheter is placed over a Benston (Cook, Bloomington, IN) or torque-control guidewire and positioned in the proximal splenic vein or at the portal vein-superior mesenteric vein confluence. Portal pressures are obtained. Injection of 15 mL of contrast per second for a total of 30 mL with digital subtraction angiography (DSA) filming at one frame per second will provide the necessary road mapping for planning left coronary vein embolization (Fig. 3–1). If varices fail to opacify, a 5F Cobra catheter can be used to catheterize the left coronary vein selectively and any other collaterals, such as short gastric varices, that arise from the splenic vein. A hockey stick, Bernstein (Cook, Bloomington, IN) or other preshaped catheter may prove necessary, depending on the angle between the varices and the portal or splenic vein. A selective injection using the DSA technique should be performed to enhance demonstration of the anatomy of the left coronary venous system and to define any left coronary vein-systemic venous shunt that could result in systemic embolization of embolic agents.18,19 After completion of the procedure, the sheath is removed as Gelfoam pledgets are inserted to embolize the transhepatic tract as the vascular sheath is removed.
Transjugular Approach
In 1975 Rosch et al20 were first to describe the transjugular approach to the portal vein for LCVE, and it was performed in humans 1 year later by Goldman et al.21 Advantages of this technique over the transhepatic approach were said to include (1) a straight-line approach for catheterization of the LCV, (2) avoidance of the peritoneal cavity, (3) decreased risk of hemoperitoneum and bile peritonitis, and (4) no risk of percutaneous leakage of ascitic fluid. Initially, this technique did not gain widespread popularity, probably because of the relative difficulty in obtaining access from the hepatic vein to the portal vein and the relative familiarity of radiologists with the transhepatic technique. The development of and recent refinements in the transjugular intrahepatic portosystemic shunt (TIPS) procedure resulted in an increasing number of LCVEs being performed from a transjugular approach. LeBerge et al22 reported performing LCVE from a transjugular approach in 34% of patients as part of their TIPS procedure.
A number of different sets designed for TIPS procedures are available from various manufacturers. We are presently using the Colapinto transjugular cholangiography and liver biopsy set (Cook, Bloomington, IN) or the Rosch-Uchida transjugular liver access set (Cook, Bloomington, IN). A 5 to 7F multipurpose catheter is first used to access one of the hepatic veins. A wedged hepatic CO2 portogram is performed in the anteroposterior (AP) and lateral planes to define the relationship between the nearest main portal vein branch and the catheterized hepatic vein. If the two vessels are in relatively close proximity, the Colapinto or transjugular needle is passed through its 9F transjugular vascular sheath and its protective sheath into the hepatic vein. Using biplane fluoroscopy, it is turned in the direction of the portal vein to be accessed. The needle is passed about 2 to 3 cm into the hepatic parenchyma. The needle is withdrawn slowly under negative pressure. When blood is aspirated, a small volume of contrast in injected to identify the vascular structure. This process is repeated carefully until the portal vein branch is located.
After the portal vein is identified, a 0.35-inch Wholey (Mallinckrodt, St. Louis, MO) or Terumo (Medi-tech, Watertown, MA) wire is advanced through the Colapinto needle and seated within the portal vein. The Colapinto catheter is gently advanced over the needle and guidewire until portal vein access is secured. A hemostatic valve can be attached to the hub of the Colapinto sheath to avoid unnecessary blood loss. A 5F catheter then can be advanced through the Colapinto sheath and into the portal vein for selective catheterization and embolization of varices.
If a TIPS is to be performed first, the procedure is modified as follows: After the Colapinto needle has been passed over the 22-gauge Chiba needle into the portal vein, the Chiba needle is removed and a 0.035-inch Wholey wire is passed down the portal vein and into the superior mesenteric or splenic vein. A 5F Van Andel (Cook, Bloomington, IN) catheter is passed through the Colapinto needle over the guidewire and into the superior mesenteric or splenic vein. The Wholey wire is exchanged for an exchange-length Super Stiff (Cook, Bloomington, IN) guidewire, and balloon dilatation and stenting of the transparenchymal tract are performed. If persistent filling of gastroesophageal varices is noted after placement of a 10-mm diameter shunt, selective embolization of the visualized varices is indicated.22 If extremely large varices are present, there may be competing flow with the TIPS, and thus a decision may be made to embolize them initially (Fig. 3–2).
Mesenteric Vein (Minilaparotomy) Approach
This procedure requires the combined efforts of surgery, interventional radiology, and anesthesiology services and provides an alternative approach for LCVE in patients who are not candidates for conventional methods or in whom more conventional methods used to control variceal hemorrhage have failed.13 The procedure is performed in the radiological suite under sterile conditions, with anesthesia available for airway control and intravenous sedation.
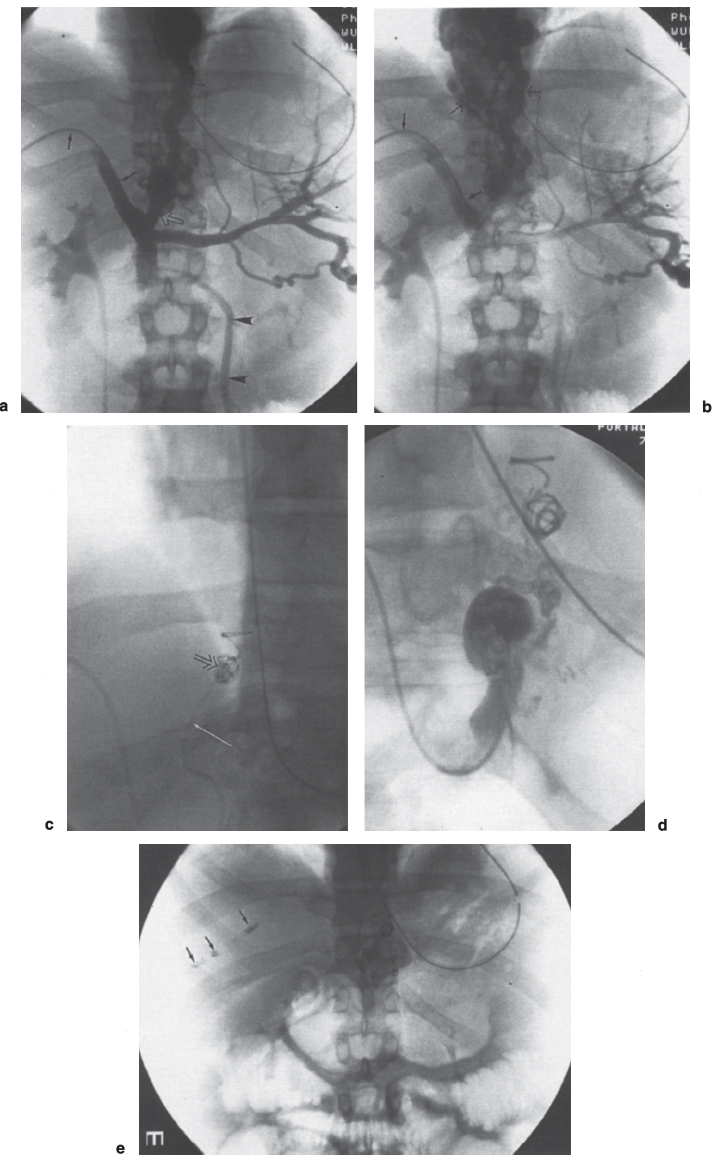
FIGURE 3–1. Percutaneous transhepatic LCV embolization. (a) Early and (b) late phase of the portal venograms obtained through a transhepatic 5F catheter (arrows) demonstrate a massive left coronary vein (open arrow) and esophageal varices (dotted arrows). Note the hepatofugal flow in the inferior mesenteric vein (arrowheads) seen on the early phase film, (c) A 5F cobrashaped catheter (arrow) is used to catheterize the left coronary vein for delivery of embolization coils (open arrow), (d) Following completion of the embolization, injection of contrast in the left coronary vein demonstrates its origin; however, there is no flow into the esophageal varices. (e) Postprocedure film demonstrates multiple embolization coils overlying the spine. In addition, multiple coils overlying the liver are noted following Gelfoam (not seen) and coil (arrows) embolization of the transhepatic tract.
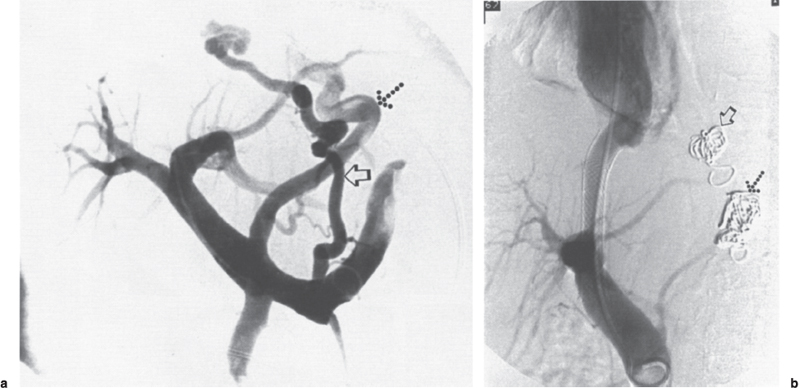
FIGURE 3–2. Seventy-two-year old woman with cirrhosis of unknown etiology and history of innumerable upper gastrointestinal hemorrhages, (a) Following a transjugular approach into the portal vein, a pigtail catheter is used to obtain a portogram showing filling of a large, left coronary vein (dotted arrow) and a short gastric vein (open arrow), which extends superiorly toward the base of the esophagus, (b) Following embolization of the LCV (dotted arrow) and short gastric vein varix (open arrow), two Wallstents were placed and balloon expanded to 8 mm, which resulted in reduction of the portal vein to right atrial gradient from 19 mm Hg to 9 mm Hg.
Local analgesia is used, a 9-cm supraumbilical midline incision is made, and a loop of distal small valve is exposed. A mesenteric vein is catheterized using the Seldinger technique, and a 7F vascular sheath is placed and advanced toward the superior mesenteric vein. Through this sheath, 5F catheters can be advanced under fluoroscopic control into the portal vein for hemo-dynamics, the splenic vein for splenic portography, and the LCV and short gastric veins for embolization. Following completion of the procedure, hemodynamics are repeated and the surgical incision is closed. The advantages of the minilaparotomy include the absence of a need to traverse a cirrhotic liver and the fact that the presence of ascites is not a contraindication. Disadvantages include the risk of wound infection, leakage of ascites through the wound, hemoperitoneum, and peritonitis.23
The original description of this combined procedure used in four patients was reported by Goldman et al.23 The 30-day survival and rebleed rate was 50%; one of two patients died secondary to recurrent hemorrhage. Durham et al13 performed this combined procedure in 15 patients and achieved a significant reduction or control of hemorrhage in 11 (73%) patients, with an average procedural time of 3.25 hours and fluoroscopy time of 38 minutes. The 30-day, 6-month, and 1-year survival rates were 60%, 33%, and 27%, respectively. Recurrent bleeding occurred in 67% of survivors but was the cause of death in only 22%.
A more extensive procedure using (1) presurgical angiographic embolization of the splenic artery (to decrease portal vein pressure), (2) minilaparotomy for LCV and short gastric vein embolization, and (3) left gastric and left gastroepiploic artery embolization in patients with recurrent bleeding (63%) after procedures (1) and (2) also has been evaluated.24 This series consisted of 50 patients, 16 of whom were Child’s class B and 34 of whom were Child’s class C patients. All the Child’s class B patients and 24 (71%) of the Child’s class C patients survived hospitalization. The 1-year survival rate was 94% (15 of 16) and 62% (21 of 34) for Child’s class B and C patients, respectively. Although rebleeding occurred in 63% (n
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



