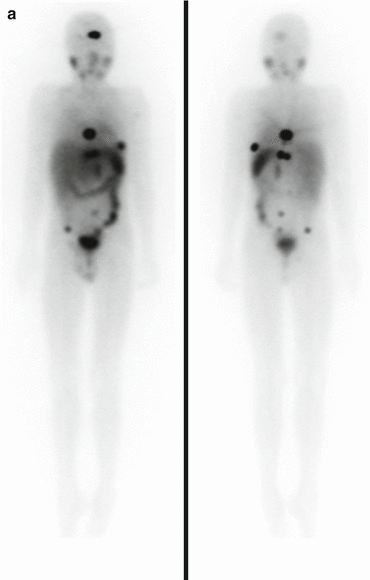
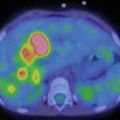
Fig. 3.1
Anterior (left) and posterior (right) planar scintigraphy of a child with papillary thyroid carcinoma performed 72 h after treatment with 3.0 GBq 131I-sodium iodide. Uptake in the left side of the thyroid bed and in multiple bilateral pulmonary metastases can be seen clearly, as well as physiological uptake and excreted activity in the bowel and bladder
If thyroid hormone withdrawal was used, liothyronine treatment should be recommenced on discharge from hospital.
Following ablation in a low-risk patient with only thyroid bed uptake, reassessment with an iodine-123 whole-body survey and SPECT/CT may be performed after a 6-month interval. If no uptake is seen and the stimulated thyroglobulin is normal, the patient is in complete remission and can be followed up clinically and with thyroid function and thyroglobulin blood tests. If residual uptake is demonstrated, further treatment is indicated. The use of neck ultrasound and stimulated thyroglobulin measurement 9–12 months after ablation is replacing the use of iodine-123 scintigraphy in low-risk patients, except where the presence of antibodies makes assessment of the thyroglobulin level difficult.
In high-risk patients, especially if nodal disease or distant deposits were identified on post-ablation imaging, further therapeutic administrations of radioactive iodine are indicated. Typically 5.5 GBq is given after a 4–6 monthly interval. With extensive disease, repeated administrations are usually recommended until no uptake is seen, and the stimulated thyroglobulin is normal.
Following completion of treatment, patients should be switched from liothyronine to levothyroxine sodium (unless they are already taking that) at slightly supra-physiological doses with the aim of keeping the TSH suppressed. Dynamic risk stratification can be used to guide the extent and duration of TSH suppression. Surveillance is based on clinical examination, supplemented if necessary by ultrasound, and thyroglobulin measurement. Sometimes interpretation of thyroglobulin levels can be complicated by the presence of anti-thyroglobulin antibodies, which may result in spuriously elevated or normal levels, depending on the assay technique used. The anti-thyroglobulin antibody titre should therefore be measured, and the result of other tests taken into account when trying to assess the significance of the thyroglobulin level. Nuclear medicine imaging is not routine unless there is a clinical suspicion. If the thyroglobulin is rising and 123-iodine imaging is normal, 18 F-FDG PET/CT can be used to see if there is iodine non-avid disease, although this is unusual. Newly discovered kinase inhibitors may improve uptake of radioiodine and are under investigation for this application.
In the event of metastatic or local relapse, discussion at an experienced MDT meeting is required, but patients can almost always be salvaged with appropriate treatment, which may include further surgery and radioactive iodine administration.
3.6 Neuroblastoma
Neuroblastoma is cancer predominantly found in babies and young children, more rarely in school age children, and exceptionally in teenagers and young adults. It is risk-stratified on the basis of age, stage and molecular pathology into low-, intermediate- and high-risk groups. The prognosis of low- and intermediate-risk groups is similarly good, although intermediate-risk patients require more intense treatments to achieve good outcomes [9].
Most patients have high-risk disease and are treated on international protocols including dose-dense platinum-based induction chemotherapy to achieve metastatic remission, surgical excision of the primary tumour, consolidation with high-dose (myeloablative) chemotherapy, external beam radiotherapy and minimal residual disease treatment with differentiating agents and immunotherapy. However, even with these very intense multimodality treatment protocols, although perhaps about one third may become long-term disease-free survivors, the majority either have poorly responding disease or relapse after achieving a remission.
Refractory and relapsed high-risk neuroblastoma is a good model for molecular radiotherapy; as there are several specific cellular targets for radiopharmaceuticals, the disease is disseminated making local treatment alone inadequate, and it is often relatively radiosensitive.
The most common type of molecular radiotherapy for neuroblastoma is iodine-131 meta-iodobenzylguanidine (mIBG), sometimes referred to as Iobenguane. 131I-mIBG, a noradrenaline analogue, is taken up into neuroblastoma cells, and other cells of neural crest origin, by a specific cell surface molecule, the noradrenaline transporter, in an oxygen- and energy-dependent active transport process. Over 90 % of patients have disease showing specific uptake of 123I-mIBG, on diagnostic imaging for staging and response assessment [17]. As a treatment, 131I-mIBG has been used clinically for around 30 years and has a variable but good clinical activity with a mean response rate of 32 % [39] (Fig. 3.2). Over time, the way in which 131I-mIBG therapy has been used has evolved. As the principal dose-limiting toxicity is haematological, investigators have sought to circumvent this by using bone marrow or more recently peripheral blood stem cell support [11, 23]. The use of haemopoietic support allows escalation of administered activity to be undertaken. As haematological toxicity is related to the whole-body dose received, safe dose escalation can be facilitated by the use of real-time whole-body dosimetry to permit a desired whole-body dose to be delivered with reasonable accuracy. There has also been interest in incorporating radiation sensitisers such as the camptothecin derivatives topotecan and irinotecan [7, 11]. Attempts have been made to improve outcomes by bringing molecular radiotherapy forward in the disease trajectory, from the relapse setting to the treatment of poor responders to induction chemotherapy, and even as the first line of treatment.
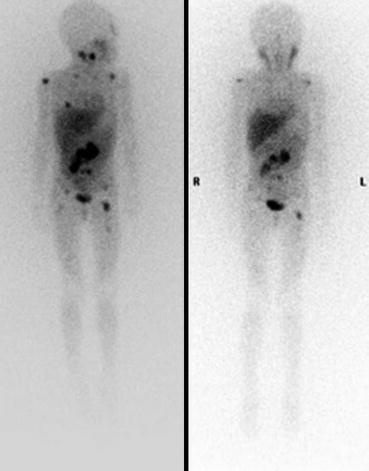

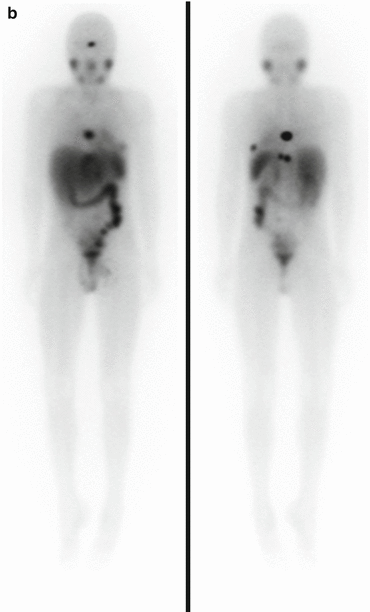
Fig. 3.2
Anterior planar 123I-mIBG scintigraphy of a child performed before (left) and after (right) 131I-mIBG therapy, showing a reduction in the size and intensity of the abdominal tumour and metastatic lesions, indicating a partial response
Although 131I-mIBG has undoubted activity against metastatic neuroblastoma, and it has been used in a range of clinical settings, its established benefit is essentially palliative, to control disease and the symptoms it causes, and perhaps to prolong life. Its role as part of potentially curative strategies remains unclear and is the subject of continuing clinical investigation. To date, no published randomised trials have been shown in a recent systematic review (Wilson et al. 2014). These are required to demonstrate superiority over other possible treatment approaches.
Practical points in the use of 131I-mIBG therapy include the need to ensure that patients are not taking concomitant medication which may interfere with uptake; the long list of such drugs can be found in the EANM guidelines [12]. As free iodine, formed by the radiolysis of 131I-mIBG, can be taken up by the thyroid, thyroid blockade is indicated. Various preparations can be used including potassium iodide, potassium iodate, potassium perchlorate and Lugol’s iodine. Despite this, there is still a risk of hypothyroidism and even thyroid cancer in long-term survivors. To avoid nausea and vomiting, anti-emetic prophylaxis should be used. There is a possibility that 131I-mIBG therapy can be associated with transient fluctuations in blood pressure, so blood pressure must be controlled before and monitored during and after treatment. [19, 40]
While the elective use of 131I-mIBG therapy is perfectly reasonable, it is preferable for patients to be enrolled in prospective clinical trials when possible. It is only through the use of sequential, well-designed clinical studies that the real place of this treatment in the management of children with neuroblastoma will be clarified.
Other molecular radiotherapy treatments have been investigated for neuroblastoma, but these have not yet gone beyond early-phase clinical trials, so must still be considered as experimental and should not be recommended as standard treatment.
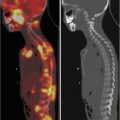
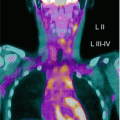
177Lu-DOTATATE, which targets the somatostatin receptor, is recognised as a standard treatment in metastatic adult neuroendocrine cancers. Its use in a small number of patients has been reported, and further clinical studies are in progress [8, 26] (Fig. 3.3a–c).
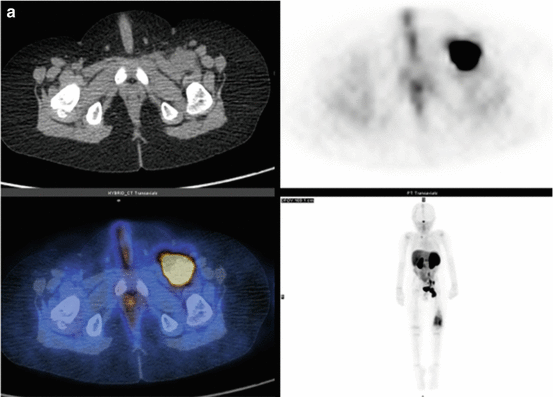
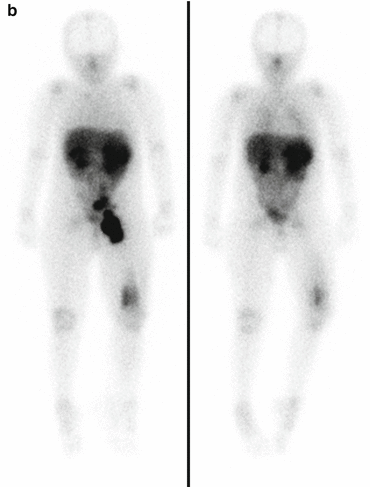
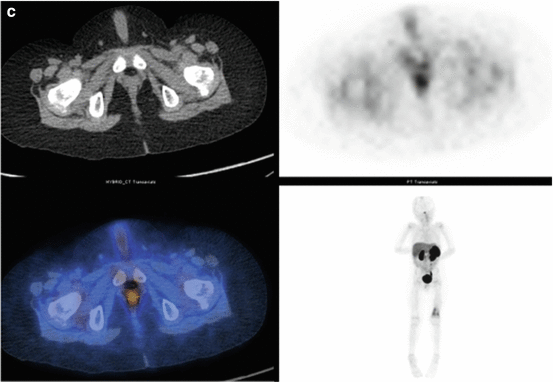

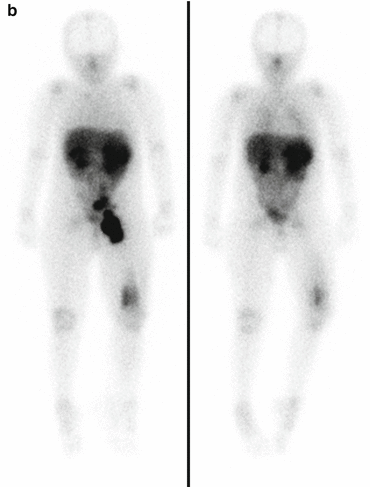

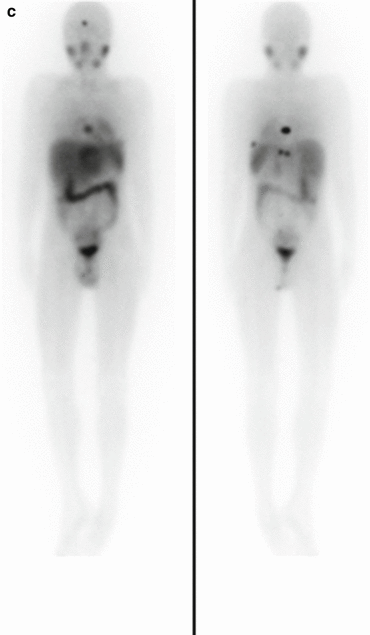
Fig. 3.3
(a) 68Ga-DOTATATE PET/CT images of a child with metastatic neuroblastoma to map disease extent prior to therapy, showing nodal deposits in the left inguinal and iliac region and a bone metastasis in the left distal femur. Axial CT image through pelvis (upper left), corresponding axial PET image (upper right), corresponding fused PET/CT image (lower left) and anterior PET maximum intensity projection image (lower right). (b) Anterior planar scintigraphy 24 h after the first administration of 177Lu-DOTATATE therapy (left) and 24 h after the second administration of 177Lu-DOTATATE therapy 2 months later (right), demonstrating an impressive response to the first course of treatment. (c) Follow-up 68Ga-DOTATATE PET/CT images of the same child 6 weeks after the two 177Lu-DOTATATE therapy administrations, showing complete response in the nodal deposits in the left inguinal and iliac region and a partial response in the bone metastasis in the left distal femur. Axial CT image through pelvis (upper left), corresponding axial PET image (upper right), corresponding fused PET/CT image (lower left) and anterior PET maximum intensity projection image (lower right)
Immunotherapy with semi-synthetic monoclonal antibodies directed against the disialoganglioside GD2 is now recognised as standard treatment [41], but although the use of radiolabelled monoclonal antibodies has been investigated preclinically [18] and clinically [20] for decades, it has not found a definite niche in the therapeutic armamentarium.
3.7 Neuroendocrine Cancers
Neuroendocrine tumours are a heterogeneous group of neoplasms which may arise at a variety of sites including the gut, pancreas, lung, thyroid gland and sympathetic nervous system. They vary also in their histological grade and malignant potential. Included in this group are phaeochromocytoma, paraganglioma, medullary carcinoma of the thyroid, carcinoid tumours, gastrinoma and more. While localised tumours are often amenable to curative surgical resection, widely metastatic tumours are incurable. Nevertheless they are very amenable to a range of treatments including molecular radiotherapy. Mostly they occur in adults, and they are very rare in children. They may be associated with a number of genetic predisposition syndromes or be familial.
Diagnostic imaging with 123I-mIBG and 68Ga-DOTATATE (or 111In-Pentetreotide) may show the extent of disease and indicate whether an attempt at molecular radiotherapy with either 131I-mIBG or peptide receptor radionuclide therapy with, for example, 177Lu-DOTATATE is merited. Patients with metastatic neuroendocrine cancers showing good uptake of both diagnostic tracers may receive both treatments sequentially. Patients with metastatic neuroendocrine cancers of adult type, unlike neuroblastoma, do not usually have widespread bone marrow infiltration and have often not been heavily pretreated with intensive chemotherapy. Sub-myeloablative activities of 131I-mIBG therapy are therefore often well tolerated without dangerous myelosuppression, and so collection of haemopoietic stem cells in advance with planned reinfusion is not necessary (Fig. 3.4a–c). The main potential toxicity of peptide receptor radionuclide therapy is renal impairment, but 177Lu-DOTATATE is less toxic in this regard than 90Y-DOTATATOC, and the use of an amino acid infusion will also significantly protect the kidneys. Expert guidance on the use of peptide receptor radionuclide therapy has been published by the EANM [1].