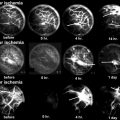
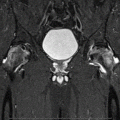
Fig. 62.1
An experimental study of the pathogenesis of femoral head deformity following ischemic osteonecrosis of the immature femoral head in the piglet model. (a) Radiographs of the central region of immature femoral heads showing a progression of the femoral head deformity in the piglet model (w weeks following the induction of ischemic necrosis). (b) A micro-CT image of a central region of the femoral head obtained at 3 weeks postischemia showing a circular area of bone resorption in the necrotic epiphysis. A radiodense, intravascular contrast material (microfil) was infused into the distal aorta before imaging to detect revascularization in the necrotic epiphysis. The arrow is pointing to a vessel with multiple small branches within the area of resorption. (c) A photomicrograph of a peripheral area of revascularization showing the increased presence of multinucleated cells (osteoclasts) and resorption of the trabecular bone (Hematoxylin and eosin staining, 20×). (d) A photomicrograph of a central area of revascularization showing fibrovascular tissue. The resorbed bone was not replaced by new bone (Hematoxylin and eosin staining, 10×) (Figures from Kim [56])
62.3 Bisphosphonate Mechanisms of Action
Bisphosphonates (BPs) are compounds which have a high affinity for bone mineral and are characterized by P–C–P bonds [16, 17]. They have two side chains that affect their binding affinity for minerals and their antiresorptive potency [18]. BPs have been used clinically for over 30 years to treat various metabolic bone disorders in the adult population. Currently, they are also being used in the pediatric population for limited clinical indications such as osteogenesis imperfecta [19]. The older BPs are nonnitrogen-containing compounds (e.g., etidronate and clodronate), whereas the newer BPs are more potent and contain nitrogen (e.g., alendronate, risedronate, pamidronate, ibandronate, and zoledronate). Once taken up by osteoclasts, nonnitrogen BPs produce toxic analogs of ATP that cause osteoclast apoptosis [20]. Nitrogen BPs act by interfering with a prenylation of small GTPase proteins and inhibiting farnesyl pyrophosphatase, an enzyme in the HMG-CoA reductase pathway, which leads to reduced resorptive activity and accelerated apoptosis of the osteoclasts [21]. While BPs have been used to treat osteoporosis, malignancy-induced hypercalcemia, Paget’s disease, and other metabolic bone disorders for many years and their clinical efficacy for these indications is well documented, the concept of using BPs to treat femoral head osteonecrosis is relatively new, and the clinical evidence supporting their use for this indication is only slowly emerging.
62.4 Experimental Evidence for Bisphosphonate Therapy
There is consistent evidence demonstrating the protective effects of bisphosphonate therapy on experimentally induced femoral head osteonecrosis. These studies can be divided into small and large animal studies [22–25].
62.4.1 Small Animal Studies
In immature rats, the effects of zoledronic acid (currently the most potent amino-bisphosphonate clinically available) were studied in a surgically induced osteonecrosis model and in spontaneously hypertensive rats that develop a Perthes-like osteonecrosis in 50 % of male animals [24, 25]. In the surgically induced osteonecrosis model, zoledronic acid treatment resulted in significantly greater trabecular bone volume and better preservation of the femoral head shape compared to the saline-treated animals which showed loss of trabecular bone volume and deformation of the femoral head [25]. The best results were obtained when zoledronic acid was administered both prophylactically (i.e., prior to the induction of osteonecrosis) and after the induction of osteonecrosis. These animals were observed to have near complete preservation of the femoral head structure. Interestingly, the animals treated with zoledronic acid after the induction of osteonecrosis also showed new bone formation in the femoral heads albeit less than that of the saline-treated animals. Similarly, treatment of spontaneously hypertensive rats with zoledronic acid increased the bone mineral density and the trabecular bone volume in the femoral head and better preserved the sphericity of the femoral head [24]. These animals also showed new bone formation in the femoral head albeit at a decreased rate than the saline-treated animals. These effects of decreased bone formation are consistent with associated reduction in bone resorption as the two processes are generally coupled.
62.4.2 Large Animal Studies
Similar finding on the inhibition of resorption was obtained using a large animal model of surgically induced osteonecrosis using another bisphosphonate, ibandronate, which is also a potent amino-bisphosphonate [23]. The infarcted femoral heads in the ibandronate-treated animals were significantly better preserved in terms of the femoral head sphericity and the trabecular bone volume. In contrast to the rat study findings, however, the bone formation in the femoral head, as assessed by histology and measurement of the osteoblast surface (%), was significantly decreased in the saline- and the ibandronate-treated animals. The difference between the rat and the piglet studies appears to stem from the rapid remodeling of the necrotic bone and restoration of new bone formation that occurs in the immature rats following osteonecrosis which is not seen in the piglet model. In the rat study, there was an absence of necrotic bone in the saline-treated animals by 6 weeks after the osteonecrosis induction [24]. This finding indicates a rapid removal of the necrotic bone and replacement by new bone which is not seen in the piglet model. The preservation of the necrotic bone following bisphosphonate therapy in the piglet model without new bone formation occurring raises a concern as the necrotic bone preserved by bisphosphonate therapy may eventually fail due to the accumulation of microcracks.
62.4.3 Experimental Studies on Local Intraosseous Bisphosphonate Therapy
It is important to recognize that local bioavailability of bisphosphonate for an avascular bone condition is different from the conditions where blood flow to the bone is not compromised. A radioactive bisphosphonate tracing study in the piglet model of ischemic necrosis showed preferential binding of 14C-ibandronate in the revascularized regions of the infarcted femoral head, whereas binding to the nonvascularized regions of the infarcted head was minimal (Fig. 62.2) [29]. This finding is not surprising since an analog of bisphosphonate, methylene diphosphonate (medronic acid, e.g., technetium MDP), is used as an imaging agent for bone scintigraphy based on its chemical property to selectively localize in the vascularized regions of the bone. After an intravenous or oral administration of bisphosphonate, it is likely that only a very small fraction of the dose actually ends up in the necrotic bone. Because of this uncertainty, multiple dosing regimens are suggested for oral or intravenous administration.
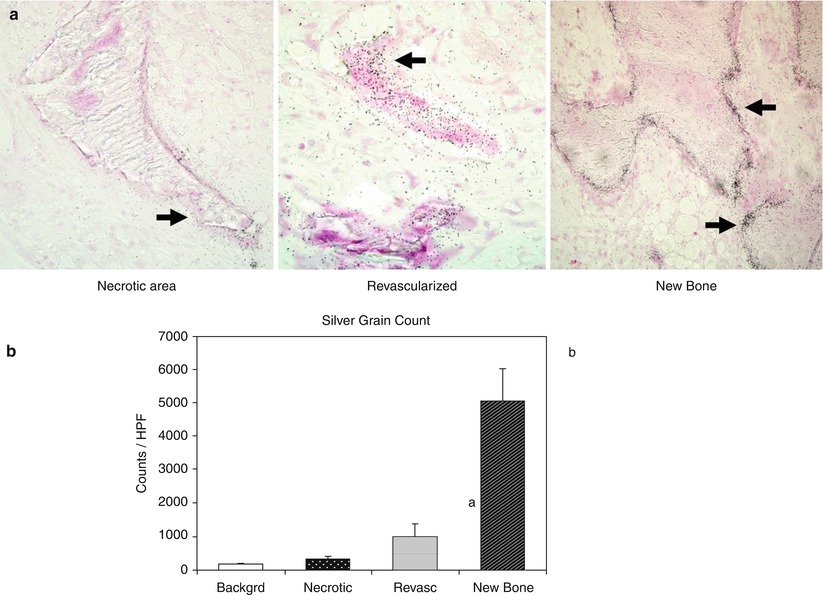

Fig. 62.2
An experimental study of bioavailability of bisphosphonate in the infarcted femoral heads following intravenous administration in immature pigs. (a) Autoradiographic images of the three regions (the region of necrotic bone, the region of revascularized marrow space, and the region of newly formed bone) found in the infarcted femoral heads at 6 weeks postischemia induction. 14C-labeled ibandronate was administered intravenously 24 h prior to sacrifice to determine its localization within the necrotic head. Arrows indicate the presence of silver grains (indicating 14C radioactivity) on the trabecular surfaces. A dense concentration of the silver grains was present on the newly formed bone but not on the necrotic bone. (b) A bar graph of mean silver grain counts obtained from the background and from the three regions found in the infarcted head at 6 weeks. a p = 0.02 vs. background and p = 0.05 vs. necrotic region, b p = 0.000001 vs. all groups (Figures from Kim et al. [29])
To overcome the limitation associated with a systemic administration, a local intraosseous administration has been investigated as an alternate route for the treatment of femoral head osteonecrosis. In the piglet model of ischemic necrosis, a single injection of 14C-ibandronate directly into the necrotic femoral heads demonstrated a wide distribution of the drug within the necrotic head with good retention at 48 h [22]. Furthermore, a single local injection effectively decreased the femoral head deformity at 8 weeks postischemia induction in the piglet model of ischemic osteonecrosis at a dose that was only 5 % of the systemic dose used in the previous study using the same large animal model (Fig. 62.3).
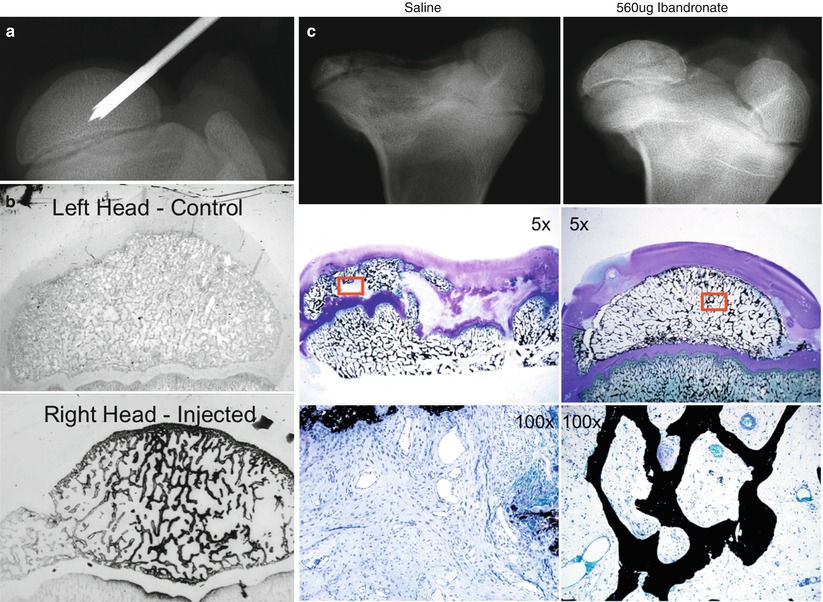

Fig. 62.3
An experimental study on the effects of local intraosseous administration of bisphosphonate in the infarcted femoral heads in immature pigs. (a) A radiograph demonstrating an intraosseous needle placed in the central region of the femoral head used to locally deliver bisphosphonate. (b) Autoradiographic sections from a control femoral head and an infarcted femoral head injected with 14C-ibandronate. A wide distribution of 14C-ibandronate, marked by diffuse staining of the trabecular bone with black silver grains, is observed in the femoral head following a single intraosseous injection. (c) A representative radiograph and photomicrographs obtained from an infarcted femoral head injected with saline (left) or 560 μg ibandronate (right) 7 weeks before the sacrifice. The femoral head structure and the trabecular bone were significantly better preserved in the ibandronate group compared to the saline group (von Kossa and McNeal’s tetrachrome staining) (Figures from Aya-ay et al. [22])
62.4.4 Experimental Studies on Combined Antiresorptive and Bone Anabolic Therapy
An ideal biological treatment for LCPD would be the one that not only controls bone resorption (antiresorptive) but also stimulates new bone formation (bone anabolic). Such treatment will improve the balance of bone resorption and bone formation which is severely perturbed in the fragmentation/resorptive stage of LCPD. While the lack of new bone formation following the administration of bisphosphonate was observed only in the piglet model and not in the rodent studies, the concern has prompted a further study to examine the effects of a combined antiresorptive and anabolic therapy on the femoral head healing following ischemic necrosis using the piglet model [30]. In this study BMP-2 was used as a bone anabolic agent and was administered with ibandronate via a single intraosseous injection into the necrotic femoral heads. In comparison to the control group, the combined therapy group had a significant decrease in the femoral head deformity and the osteoclast number and a significant increase in the trabecular bone volume and the osteoblast surface (Fig. 62.4). In comparison to the local ibandronate treatment group, the combined treatment group also had a significantly higher osteoblast surface, suggesting an increased bone formation as a result of adding BMP-2. One cautionary finding from this study was the presence of heterotopic ossification in the hip joint capsule which was postulated to be due to a leakage of BMP-2 into the joint during and following the local injection procedure. Further studies to determine whether the heterotopic ossification can be prevented by lowering the dose of BMP-2, changing the injection technique, and using another type of BMP are pending.
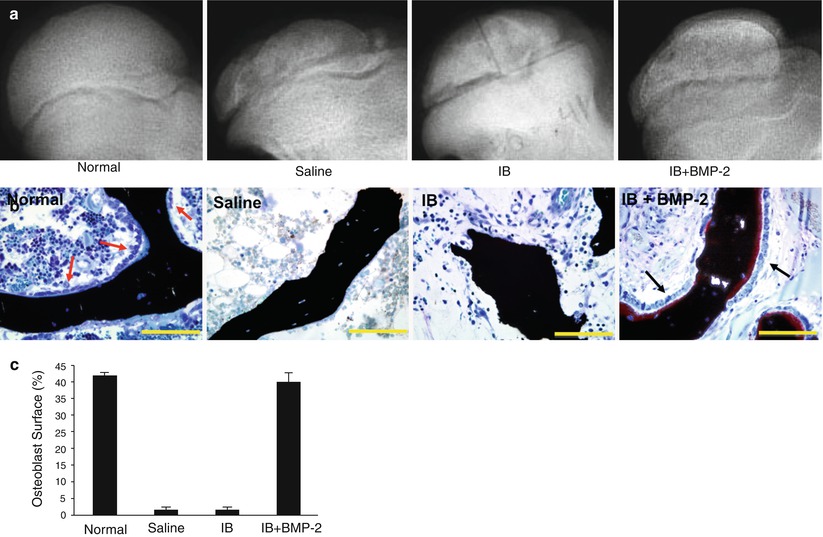

Fig. 62.4
An experimental study on the effects of a combined local bisphosphonate and BMP-2 therapy on the infarcted femoral heads of immature pigs. (a) Radiographs of the animals treated with intraosseous saline, ibandronate (IB), or IB plus BMP-2. A single injection of respective agent(s) was rendered 1 week following the induction of ischemic osteonecrosis. (b) Photomicrographs showing a presence of osteoblasts on the surface of the trabecular bone in the IB + BMP-2 group. The femoral heads from the saline and the IB groups had a lack of osteoblasts on the trabecular surfaces. (c) A bar graph showing significantly greater osteoblast surface (%), an indicator of bone formation, in the IB + BMP-2 group compared to the saline and the IB group (Figures from Vandermeer et al. [30])
62.5 Clinical Evidence
At this time there is no direct clinical evidence to support the use of bisphosphonate therapy to prevent femoral head deformity in LCPD. While the use of bisphosphonate therapy for LCPD has been reported from one center in Australia [31, 32], clinical studies assessing the efficacy of this treatment have not been performed. However, a randomized clinical trial is underway in Australia comparing intravenous administration of zoledronic acid to a standard care (weight-bearing restriction and current treatments) for LCPD (Clinical Trial Registration ACTRN12610000407099). While direct clinical evidence supporting bisphosphonate therapy for LCPD is lacking, a limited number of studies on other forms of childhood femoral head osteonecrosis and adult femoral head osteonecrosis have been reported.
62.5.1 Evidence in Adult Femoral Head Osteonecrosis
In the adult population, at least seven clinical studies on patients treated with bisphosphonate for nontraumatic osteonecrosis of the femoral head are available [33–37]. In general, these studies show some beneficial effects of bisphosphonate therapy on pain, function, and preservation of the femoral head [33–36]. Among these studies, one of them was a randomized clinical trial involving 40 patients (54 hips) with Steinberg IIC and IIIC hips (i.e., pre-collapsed femoral heads with >30 % head involvement on the MRI) [35]. In the study, only 2 out of 29 hips required total hip replacement following oral alendronate therapy (70 mg weekly dosing) for 6 months compared to 19 out of 25 hips in the non-treatment group at the minimum follow-up of 2 years. The short-term results are impressive; however, the authors observed no evidence of reduction or resolution of the necrotic area of the femoral head on radiographs or MRI at the final follow-up suggesting that longer follow-up studies are needed. More recent multicenter, prospective, randomized, double-blinded, placebo-controlled study, however, showed no difference between the alendronate treatment and the placebo group in terms of the radiographic or MRI results at 2-year follow-up [38]. For both groups the rate of total hip replacement at 2-year follow-up was low, 4 out of 32 hips in the alendronate treatment group and 5 out of 33 hips in the placebo group. These low event rates raise the question of whether the patients included in the study had clinically significant femoral head osteonecrosis as the clinical outcome of large femoral head osteonecrosis is generally poor. Further, with this low event rate, it is estimated that a sample size over 200 patients in each group would be required to reach 80 % power.
Among the adult studies, the study by Agarwala et al. had the longest follow-up [34] (up to 8 years). In their clinical series (level 4 evidence) that included 294 patients (395 hips) in Ficat and Arlet stage 1–3, oral alendronate therapy (10 mg daily) produced the best results when the treatment was initiated in the early stage of femoral head osteonecrosis (stage 1). At the mean follow-up of 4 years, 2 % (4 out of 215), 8 % (10 out of 129), and 33 % (17 out of 51) of the patients in stage 1, 2, and 3, respectively, had a total hip replacement. In terms of radiographic progression, 13 % (27 out of 215) of the patients in stage 1 and 56 % (72 out of 129) of the patients in stage 2 had a radiographic evidence of collapse. Major limitations of this study were that the extent of the femoral head involvement was not assessed and no controls were included in the study to determine the efficacy of the treatment.
62.5.2 Evidence in Childhood Femoral Head Osteonecrosis
In children, bisphosphonate therapy has been used to treat LCPD [31, 32], traumatic osteonecrosis [39], and osteonecrosis as a complication of chemotherapy for childhood leukemia and non-Hodgkin lymphoma [40–42]. The numbers of patients treated with bisphosphonate therapy in these studies are small, and the level of evidence is restricted to case reports, case series, or observational studies. The follow-up duration of these studies has also been short. In a small number of patients with LCPD (n = 17), the systemic effects of intravenous bisphosphonate have been reported [31]; however, its effect on the preservation of the femoral head has yet to be reported. A prospective case series of traumatic osteonecrosis due to unstable SCFE, hip fracture, or dislocation in adolescents showed that the patients treated with intravenous bisphosphonate (pamidronate or zoledronic acid) did better than expected from historical controls at the minimum follow-up of 2 years [39]. Nine out of 17 patients had a spherical femoral head and 14 out of 17 patients were pain free. The mean Harris hip score, Iowa hip rating, and global PODCI score were 91.2, 92.1, and 91.5, respectively. This study, however, did not have a control group. In an observational study of 17 patients with osteonecrosis as a complication of chemotherapy for childhood leukemia [41], improvements in the pain scores, analgesic requirement, and function were found in the nine patients who received bisphosphonate therapy, while seven of eight patients who did not receive bisphosphonate therapy showed clinical deterioration. A radiographic benefit of the therapy, however, could not be demonstrated. Another small study of childhood leukemic patients found a reduction of pain and increased mobility in four of the six patients treated with pamidronate for 2 years [42]. Pamidronate therapy, however, did not prevent late bone collapse as three of the six patients had a progression and required hip replacement. A more recent study of pediatric oncology patients treated with zoledronate found that only 25 % patients have a spherical femoral head and pain free at the end of treatment, 25 % had hip arthroplasty, and 50 % had ongoing pain and disability that was expected to lead to hip arthroplasty [43].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree