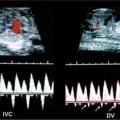
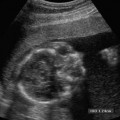
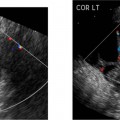
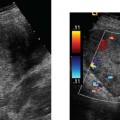
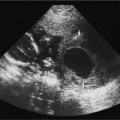
19 Diabetes Mellitus and Pregnancy It has been estimated that diabetes mellitus affects between 1 and 2 million women of childbearing age in the United States.1 The management of this disease has evolved from an attempt to improve maternal survival in the 1900s and fetal survival in the middle part of the century to the prevention of fetal morbidity in the 1990s. The diagnosis and management of the maternal complications of diabetes mellitus are numerous and complex and will not be addressed in this discussion. Rather, the fetal complications, including fetal malformations; growth disturbances, including intrauterine growth restriction; macro-somia; and prematurity will be discussed.2,2 Pregnancy itself has often been referred to as diabetogenic. In fact, this is only partially true. In the first trimester, estrogen- and progesterone-induced pancreatic β-cell hyperplasia results in increased insulin production and a lowering of fasting blood sugar in both diabetic and nondiabetic pregnancies. In the second and third trimesters, the levels of human placental lactogen, a polypeptide hormone that is an insulin antagonist, rise. In addition, prolactin, cortisol, estrogen, and progesterone also exert a contrainsulin effect. Diabetes mellitus occurring during pregnancy has been traditionally categorized according to the White classification. The basis of this system relied upon the fact that the severity of diabetes can be quantified and is directly related to both maternal and perinatal outcomes. The White classification grouped patients into classes A through F on the basis of the type of therapy administered, the duration of maternal diabetes before pregnancy, and the presence or absence of maternal vascular complications. Class A refers to those patients with gestational diabetes, whereas classes B, C, and D include patients with diabetes mellitus predating their pregnancies. Classes F, R, and H represent those diabetic women with evidence of vascular disease. Although the White classification has been useful for identifying women at risk for an adverse outcome, it has recently fallen into disuse. As our understanding of diabetes and pregnancy has improved it has become clear that most of the fetal risk is related to the time during pregnancy when diabetes is present, the degree of metabolic control achieved with therapy, the presence of maternal vascular complications, and the presence of medical complications, such as hypertension and urinary tract infections. A more modern classification divides patients into two large groups: those whose diabetes antedated pregnancy (pregestational diabetes) and those whose diabetes was first diagnosed during gestation (gestational diabetes).4 Fetal risks in the former group include those derived from maternal metabolic abnormalities during the first trimester (birth defects and spontaneous abortion), as well as during the second and third trimesters. (e.g., macrosomia, hyperinsulinemia, and stillbirth). Fetal risks in women with gestational diabetes are primarily derived from metabolic abnormalities in the second and third trimesters. In addition to the above classifications, diabetes mellitus may be characterized as type I, insulin-dependent diabetes mellitus (IDDM) and type II, noninsulin-dependent diabetes mellitus. Type I diabetes is the ketotic-prone form of the disorder, whereas type II is the so-called maturity-onset, nonketotic-prone form of the disease. Through the use of fetal biometry, ultrasound is able to determine fetal age, weight, and growth. The accurate determination of fetal gestational age is important in the diabetic patient to aid in the timing and interpretation of maternal serum α-fetoprotein (AFP) levels, in the timing of third-trimester amniocentesis and delivery, and in the evaluation of fetal growth. In patients in whom the menstrual history is uncertain, first-trimester ultrasound may be necessary to establish the gestational age to aid in the timing of either maternal serum AFP testing or amniocentesis. Accuracy within 4 to 7 days may be achieved in most cases. As will be discussed later, although the accuracy for estimating gestational age is excellent, the ability to detect many significant fetal malformations is poor in the first trimester. A sonogram performed between 18 and 20 weeks of gestation will still have an accuracy of ± 1 week for defining gestational age, and greater than 90% likelihood of detecting serious fetal malformations. Perhaps the most significant fetal complication of pregnancy encountered by pregestational diabetic women is a significantly increased risk of fetal congenital malformations. An association between diabetes mellitus and congenital malformations was suspected as early as 1885, when it was reported that infants of diabetic mothers had an increased incidence of congenital malformations.5–7 In a study done between 1926 and 1963, 6.4% of infants of diabetic mothers had major congenital malformations compared with 2.1% in the control group.8 Virtually all other studies done subsequently have verified the increased incidence of congenital malformations in this population to be three to four times that of controls.5,9,10 Congenital malformations account for 35 to 40% of all perinatal deaths and are the leading cause of death among infants of diabetic mothers. Despite routine widespread morphological ultrasound scans, the rates of perinatal death from congenital anomalies in diabetic pregnancies have not changed over the last decade.11,2 A study by Wong et al demonstrated that within the same institution the detection rate of congenital anomalies for diabetic women was significantly lower than that for the general population (30% vs 73%).11 The authors postulated several reasons that might account for this difference. First, 30% of the major anomalies in the diabetic group were considered not detectable by current ultra-sound technology at the gestational age when the scan was performed. This was higher than in the low-risk group. However, after excluding these anomalies, the detection rate was still lower than in the low-risk group (42% vs 86%). Second, the diabetic women were more obese, with an average body mass index (BMI) of 29 kg/m2 versus 23 kg/m2 in the nondiabetic group. It is well known that obesity is significantly associated with poor ultrasound imaging.13 In one study, image quality was considered unsatisfactory in 37% of the diabetic women. The detection rate of congenital anomalies may improve with technological advancements in ultrasound, such as harmonic imaging, which is available in most new ultrasound machines. Likewise, the use of transvaginal sonography at 14–16 weeks may overcome problems with excess subcutaneous tissue.11 The malformations most commonly observed in fetuses of diabetic mothers are cardiac defects, neural tube defects, caudal regression syndrome (sacral agenesis), and renal abnormalities (Fig. 19–1, 19–2). Although the focus of many clinical centers is on the detection of neural tube disease in pregestational diabetics, cardiac abnormalities are by far the most common abnormalities in these patients. The reported incidence of cardiac abnormalities is increased to between 2 and 4% in pregestational diabetics as compared with the incidence of 0.8% in the general non-diabetic population.1,2 The abnormalities most commonly seen are conotruncal abnormalities, such as transposition of the great vessels (Fig. 19–3), truncus arteriosus (Fig. 19–4), tetralogy of Fallot (Fig. 19–5), and ventricular septal defects (Fig. 19–6). Many of the abnormalities will not be detected with just the standard four-chamber view of the heart. In a recent study by Smith et al,15 the sensitivity of ultrasound for detecting an abnormal heart increased from 73% with the four-chamber view (Fig. 19–7) to 82% with the addition of the aortic outflow tract view (Fig. 19–8). There were two false-negative and no false-positive diagnoses. In their study, when the four-chamber view and outflow tracts appeared normal, additional views, such as the ductal and aortic arches, did not detect a cardiac defect. Additional cardiac abnormalities that may be seen in the diabetic patient include cardiomyopathy (Fig. 19–9) and aortic coarctation. Clearly, if a gravid diabetic patient is to have an ultrasound evaluation for the purpose of detecting morphological abnormalities, it should include an evaluation of the fetal heart by an experienced fetal and/or pediatric echocardiographer. Neural tube defects are increased in the diabetic population. The incidence of neural tube defects is said to be 19.5 per thousand in diabetic mothers compared with one to two per thousand in the general population.12 Anencephaly (Fig. 19–10) as well as myelomeningoceles (Fig. 19–11) may be seen in this population. Caudal regression syndrome (sacral agenesis) is wrongly often assumed to be an abnormality seen only in the diabetic population. Caudal regression, in which there is hypoplasia of the sacrum and lower extremities, was first described in 1964 as being more common in infants of diabetic mothers.13 Associated anomalies that may be seen at birth are fusion of the lower limbs (sirenomelia), absence of the bladder, imperforate anus, absence of external genitalia, renal agenesis (Fig. 19–12), hypospadias (Fig. 19–13), and single umbilical artery. As already stated, the abnormalities that form the basis of caudal regression syndrome may be seen in nondiabetics as well.
Pathophysiology and Classification
Ultrasound Evaluation
Fetal Malformations
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree