
Diagnosis and Management of Venous Malformations
Vascular anomalies constitute some of the most difficult diagnostic and therapeutic enigmas encountered in the practice of medicine. The clinical presentations are extremely protean and can range from an asymptomatic birthmark to life-threatening hemorrhage. Correctly attributing any of the extremely varied symptoms to a vascular malformation can be challenging to the most experienced clinician. Compounding this problem is the extreme rarity of these vascular lesions. If a clinician sees one such patient every few years, it is extremely difficult to gain a learning curve to diagnose and optimally treat patients who have vascular anomalies. Typically, these patients bounce from clinician to clinician only to experience disappointing outcomes, complications, and recurrence or worsening of their presenting symptoms. Vascular malformations include high-flow malformations and low-flow malformations. The high-flow malformations include arteriovenous malformations, congenital arteriovenous fistula, posttraumatic arteriovenous fistula, and acquired arteriovenous fistula. Low-flow lesions include venous malformations, lymphatic malformations, and mixed lesions. This chapter is devoted to the diagnosis and management of venous malformations.
Before the advent of magnetic resonance imaging (MRI), contrast-enhanced computed tomography (CT) scanning, and, rarely, ultrasound imaging, were used. Most often, patients were evaluated by arteriography and venography. Later, closed-system venography and direct puncture venography were used to evaluate these postcapillary abnormal venous spaces more accurately.1,2 Currently, MRI is the mainstay in initial diagnosis as well as to assess endovascular therapy at followup.3–6 Color Doppler imaging (CDI) is also assuming a large role in the initial diagnosis and later follow-up of venous malformations.7,8
Initially, venous malformations were treated by surgeons. Because of the significant blood loss that occurred during surgery, partial surgical resections were the rule. Partial resections could cause an initial good clinical response; however, with time, the patients’ presenting symptoms recurred or worsened at follow-up. Further, the venous malformation rarely was limited to a subcutaneous position, and more often than not it involved multiple muscular structures as well as neurovascular compartments. Thus, direct puncture and ethanol embolotherapy have emerged as primary modes of therapy in the management of these abnormal slow-flow vascular lesions,9–15 which led to enhanced care of these problematic patients.
Because the clinical and angiographic manifestations can be extremely varied, venous malformations, and vascular malformations in general, always have been difficult to classify. Moreover, a vast array of descriptive terms have been given to impressive clinical examples in hopes of distinguishing them as distinct entities or syndromes. Confusion in the literature has resulted, with miscategorizations and, thus, suboptimal treatment of these complex lesions. Some of the confusing terms include congenital arteriovenous aneurysm, interosseous anomaly, cirsoid aneurysm, serpentine aneurysm, capillary telangiectasia, angioma telangiectaticum, angioma arteriala racemosum, angioma simplex, angioma serpingiosum, nevus angiectoides, hemangioma, lymphangioma, hemangiolymphangioma, verrucous hemangioma, capillary hemangioma, cavernous hemangioma, venous angioma naevus flammeus, and others. Based on the landmark research of Mulliken et al,17 a rational classification of hemangioma and vascular malformations has evolved that should be incorporated into modern clinical practice. This classification system, based on endothelial cell characteristics, has removed much of the confusion in terminology present in the literature today. Once all clinicians understand and use this important classification system, ambiguity and confusion will be removed, and all clinicians will speak a common language.16–20
 Theoretic Embryologic Origins
Theoretic Embryologic Origins
In the embryo, the primitive mesenchyme is nourished by an interlacing system of blood spaces without distinguishable arterial and venous channels. As the embryo matures, the interlacing system of blood spaces becomes more differentiated by partial resorption of the primitive elements and formation of more mature arterial and venous elements within an intervening capillary bed. The classically outlined sequence of events includes (1) the undifferentiated capillary network stage; (2) the retiform developmental stage characterized by coalescence of the original equipotential capillaries into large interconnecting plexiform vascular spaces without an intervening capillary bed; and (3) the final developmental stage, characterized by the resorption of the primitive vascular elements and the formation of mature arterial, capillary, venous and lymphatic elements. Failure or orderly resorption of arrests in development in these embryologic primitive vascular spaces results in the persistence of immature vascular anomalies. Retention of primitive retiform elements is the theoretic origin of congenital venous malformations that are retained in the fetus and presented at birth.21–24 As Reid23 stated, “In view of the common development on each side of the vascular tree, and in view of the enormous constructive and destructive changes necessary before the final pattern of the vascular tree is reached, it is a marvel not that abnormal congenital communications occasionally, or rarely, occur, but that they do not occur more often.”
 Classification of Hemangiomas and Vascular Malformations
Classification of Hemangiomas and Vascular Malformations
Pediatric hemangioma and vascular malformations have been classified by Mulliken, Glowacki, and co-workers after they conducted research into the characteristics of endothelial cells, the numbers of mast cells present, and the characteristics of endothelial cells in vitro.16–20 It must be emphasized that pediatric hemangiomas are tumors that are usually not present at birth, clinically manifest themselves sometime within the first month of life, and exhibit a rapid growth phase within the first year. More than 90% of these tumors spontaneously regress to near complete resolution by the time the patient is aged 5 to 7 years. Hemangiomas are the most common tumors of infancy and occur with a reported incidence of 1 to 2.6%.20,25 Pediatric hemangiomas in the proliferative phase are characterized by rapid growth, significant endothelial cell hyperplasia forming syncytial masses, thickened endothelial basement membrane, ready incorporation of tritiated thymidine into the endothelial cells, and the presence of large numbers of mast cells. After this period of rapid expansion in the proliferative phase, hemangiomas stabilize in size and may grow commensurately with the child. Because of the complex nature of hemangioma, the proliferative phase may continue in parts of the tumor as the involution phase starts in other parts of the tumor, but eventually involutive aspects begin to dominate. Involuting hemangiomas show diminished endothelial cellularity and replacement with fibrofatty deposits, exhibit a unilamellar basement membrane, demonstrate no uptake of tritiated thymidine into endothelial cells, and have normal mast cell counts.16–18
Vascular malformations, which include venous malformations, are vascular lesions that are present at birth and grow commensurately with the child. Vascular malformations demonstrate no endotheolial cell proliferation, contain large vascular channels lined by flat endothelium, have a unilamellar basement membrane, do not incorporate tritiated thymidine into endothelial cells, and have normal mast cell counts. They may be formed from any combination of primitive arterial, capillary, venous, or lymphatic elements with or without direct arteriovenous (AV) shunts. Vascular malformations are true structural anomalies resulting from errors in vascular morphogenesis. Trauma, surgery, or hormonal influences caused by birth control pills, puberty, and pregnancy may cause vascular malformations to enlarge and become more symptomatic.20–26
Vascular malformations are categorized into arterial, capillary, venous, or lymphatic vascular elements that are malformed. The term hemangioma should be reserved solely for the previously described pediatric tumor, which is usually not present at birth, becomes manifest within the first month of life, and exhibits a rapid proliferative phase followed by an involutive phase. The older terms describing adult conditions, such as cavernous hemangioma, hepatic hemangioma, extremity hemangioma, vertebral body hemangioma, venous angioma, and intramuscular hemangioma, should be replaced with the term venous malformation. Because hemangioma is universally absent by the age of 10 years, it cannot exist in the adult patient. Therefore, we should exclude all terms such as hepatic hemangioma, vertebral body hemangioma, and cavernous hemangioma, to describe lesions that exist in the adult population. These lesions are truly malformed veins and should be stated as such in our literature.
Eponyms have further clouded and confused the nomenclature of hemangiomas and vascular malformations in the literature. Maffucci’s syndrome (Kast’s syndrome) has been defined as a condition in which the patient has multiple enchondromas and coexisting hemangiomatosis.27 The term hemangiomatosis should be replaced with venous malformations. The Riley-Smith syndrome is characterized by macrocephaly, pseudopapilladema, and multiple hemangiomas.28 The term hemangioma should be replaced with venous malformation. The Riley-Smith syndrome, the Proteus syndrome,29 and Bannayan’s syndrome30,31 are probably a spectrum of similar congenital vascular anomalies. Gorham syndrome, Gorham-Stout syndrome, and Trinquoste syndrome are similar entities describing an osteolysis (disappearing bone disease) caused by an underlying hemangiomatosis.32 The term hemangiomatosis should be replaced by intraosseous vascular malformation. Another confusing group of eponyms (Klippel-Trenaunay syndrome, Parkes-Weber syndrome, Klippel-Trenaunay-Weber syndrome, Klippel-Trenaunay-Weber-Rubashov syndrome, giant limb of Robertson, nevus vasculosus osteophyperrtrophycus, nevus verrucosus hypertrophycans, osteohypertrophic nevus flammeus, angioosteohypertrophy syndrome) all describe a congenital entity characterized by unilateral limb hypertrophy; cutaneous port wine stains; lymphatic malformations; a normal, hypoplastic, or atretic deep venous system; occasional extension of the vascular malformation into the trunk; a retained embryonic lower-extremity lateral venous anomaly (Servelle’s vein); and increased subcutaneous fat in the affected limb. The lower extremity is more commonly affected than the upper extremity. There may be the coexistence of multiple arteriovenous fistulas as well.33,34
These examples are but a few of the confusing terms used in the literature and in clinical practice. Use of this modern classification system could eliminate the current confusion, and all clinicians finally can speak the same language. Accurate terminology will lead to precise identification of clinical entities and to enhanced patient care. The remainder of this chapter uses this modern classification system originated by Mulliken, Glowacki, and co-workers.33,34
 Concepts in Patient Management
Concepts in Patient Management
Vascular malformations are congenital lesions that are present at birth, whether or not they are evident clinically. They do grow commensurately with the child. A thorough clinical examination and history usually can establish the diagnosis of hemangioma or vascular malformation. Hemangiomas are usually not present at birth and initially have a bright scarlet skin lesion that gradually deepens as the mass enlarges. Vascular malformations have a persistent color, usually more bluish, depending on the dominant arterial, capillary, venous, or lymphatic component present. Evaluating for skeletal abnormalities, abnormal veins, arterial abnormalities, pulsatility or nonpulsatility of a lesion, whether the lesion swells when dependent and flattens when elevated, disparity of limb size, and whether reflex bradycardia occurs (Nicoladoni-Branham test), along with neurologic evaluation and a good history frequently can diagnose a hemangioma or categorize a vascular malformation.
Ultrasound Evaluation
Color Doppler imaging has proven to be an excellent diagnostic tool in the initial evaluations of patients with venous malformations. It can accurately determine whether a high-flow or low-flow lesion is present. CDI with spectral analysis gives accurate information for categorizing a high-flow or low-flow malformation. A high-flow malformation has in-flow arteries that typically demonstrate high velocity and a low-resistance waveform. Low-flow malformations demonstrate normal arterial flow volumes as well as normal high arterial resistance in the arteries that supply the locale where the venous malformation is present. Lower resistance waveforms may be present in the intramuscular type of venous malformations. CDI further characterizes the venous malformation as demonstrating slow to stagnant flow within the malformation itself.
Low-flow malformations vary widely in appearance on B-mode imaging, depending on the relative proportions of dilated postcapillary channels in the vessel wall. A vessel wall may appear relatively echogenic. If the luminal components predominate, multiple cystic spaces or even isolated dilated varices may be present. A continuous spectrum in different areas of the venous malformation may have different sonographic characteristics. Likewise, the degree of compressibility varies, depending on the composition of the venous malformation. If luminal components predominate, the lesion may be almost completely compressible. Echogenic venous malformations in which the wall components predominate (with smaller luminal elements) are generally less compressible. On B-mode imaging, slow to stagnant flow is identified. Flow is frequently too slow to demonstrate without augmentation on even the most sensitive settings with the best color Doppler equipment. We found “autoaugmentation” to be the most effective means of demonstrating flow within venous malformations. Compression of the venous malformation followed by sudden release of the compression resulting in refilling of the malformation with more rapid than normal flow from the surrounding uncompressed areas does cause a Doppler shift. The fact that arteries in the area of the malformation have normal high-resistance velocities correlates well with the fact that this is not an arterial abnormality but truly a postcapillary venous lesion.
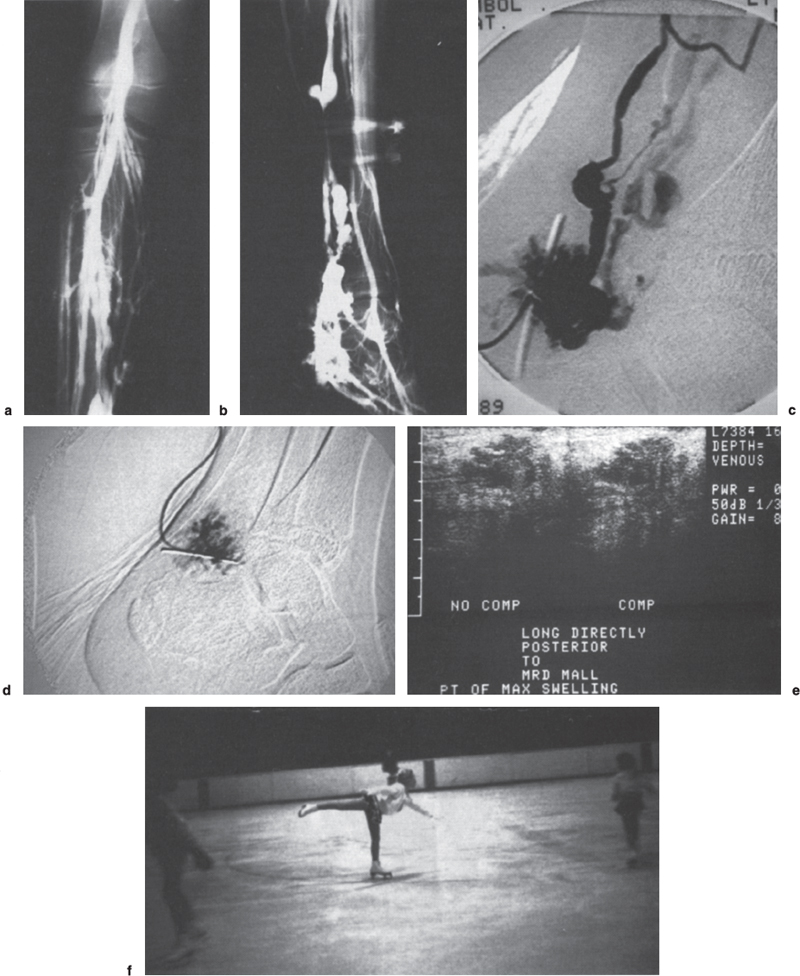
FIGURE 13–1. (a) Anteroposterior closed-system venogram showing abnormal venous malformation along the posterior tibial distribution. (b) Lateral view closed system venogram of left lower extremity demonstrating extensive venous malformation along the posterior tibial venous distribution. (c) Lateral direct puncture digital subtraction angiography (DSA) into venous malformation along the posterior tibial distribution. (d) Postembolization direct-puncture angiogram showing thrombosis of the abnormal vascular compartment. (e) Postdirect puncture embolization ultrasound showing thrombosis and noncompressibility of the abnormal vascular compartment. (f) Patient skating well and competing at 4-year follow-up without recurrence of symptoms.
For monitoring the treatment of malformations, CDI can be useful and can confirm thrombosis within the malformation as demonstrated by noncompressibility (Fig. 13–1
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



