Diffusion weighted imaging (DWI) is a useful diagnostic tool for central nervous system (CNS) infectious disease, among other pathologies.
Meningitis, regardless of its cause, can present subarachnoid hyperintensities on DWI. Complications of meningitis, such as infarctions, venous thrombosis, empyema, ventriculitis, and abscess also present characteristic findings on DWI.
Pyogenic abscess usually presents as a hypointense rim on T2-weighted imaging, a peripheral enhancement after contrast administration, and a hyperintense core on DWI with reduced apparent diffusion coefficient (ADC). Fungal or tuberculous abscesses may present variable signal intensities on DWI and are not always hyperintense as pyogenic abscesses are. Fungal abscesses are commonly multiple, and their borders are loculated or crenated.
DWI can identify early findings of herpesvirus infection with areas of restricted diffusion on the temporal and frontal lobes, insula, and cingulate gyrus.
In immunocompromised patients, progressive multifocal leukoencephalopathy (PML) can present as a leading edge of hyperintensity on DWI, sometimes with restricted diffusion. Toxoplasmosis lesions do not present with restricted diffusion.
DWI is very useful in patients with Creutzfeldt–Jakob disease because the lesions present as hyperintense signal on DWI and are better seen than with other imaging sequences.
8.1 Clinical Applications
8.1.1 Introduction
Neuroimaging is an essential tool in the diagnosis and therapeutic planning of central nervous system (CNS) infectious disease. The introduction of diffusion weighted imaging (DWI) has improved the diagnosis of certain infectious process. DWI can provide additional information to conventional magnetic resonance imaging (MRI) sequences that allows a better understanding of the pathophysiology as well as more precise and early diagnosis in many infectious diseases. DWI is an important imaging tool for the diagnosis of many infectious lesions, such as encephalitis, abscess, ventriculitis, empyema and other complications of infectious processes, such as infarcts due to vasculitis or venous thrombosis. In some instances diffusion tensor imaging (DTI) may help follow up lesion response after proper treatment is initiated, and may better evaluate its sequelae. This chapter discusses the main groups of etiologic agents: bacterial, viral, fungal, parasitic, and prion diseases, and the contributions of DWI and DTI in their diagnosis, therapeutic strategies, and follow-up.
8.1.2 Brief Overview of Infectious Diseases and DWI and DTI
Bacterial Infections
Bacterial infections require prompt diagnosis and treatment. The infectious process can be restricted to the meninges and cerebrospinal fluid (CSF) compartment (meningitis, ventriculitis) or can spread to the brain parenchyma (cerebritis, brain abscess). It can also be located within the meningeal layers (empyema).
Meningitis is defined as the inflammation of the membranes surrounding the brain and spinal cord. Usually the diagnosis is based on clinical findings and CSF analysis. Imaging can be required before lumbar puncture to rule out increased intracranial pressure and to detect complications of meningitis.1
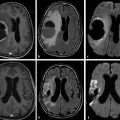
In meningitis, computed tomography (CT) is the most commonly used imaging diagnostic tool, regardless of underlying bacteria. Magnetic resonance imaging (MRI) is not usually required for uncomplicated meningitis, but MRI is superior to CT for depicting meningitis complications, such as vasculitis and empyema. MRI can show meningeal abnormalities on fluid-attenuated inversion recovery (FLAIR) or postcontrast T1-weighted images (the authors reserve the use of postcontrast FLAIR imaging for specific conditions, such as infectious and carcinomatous meningitis). DWI can demonstrate subarachnoid hyperintensities as well as complications of meningitis, such as acute infarcts due to septic vasculitis and venous thrombosis. Subarachnoid DWI hyperintensity in meningitis is attributed to proteinaceous exudate with inflammatory cells and debris (▶ Fig. 8.1).2
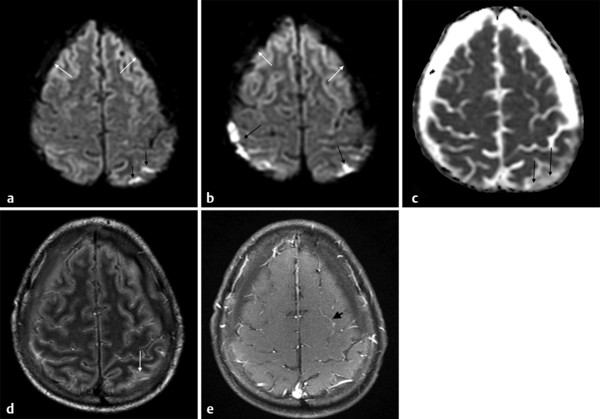
Fig. 8.1 Streptococcus pneumoniae meningitis with hyperintensity on diffusion weighted imaging (DWI). (a,b) Axial DWI shows hyperintense parietal sulci (black arrows) and hypointense subdural effusions bilaterally (white arrows). On the apparent diffusion coefficient (ADC) map, (c) the parietal sulci ADC (black arrows) is reduced compared to CSF, although not reduced compared to the parenchyma. In the subdural collections the ADC is close to cerebrospinal fluid (CSF). (d) The axial enhanced fluid-attenuated inversion recovery image also shows hyperintensity in parietal sulci (white arrow) and the bilateral subdural effusion similar to CSF. (e) The contrast-enhanced axial T1-weighted image shows subtle leptomeningeal enhancement in some sulci (black arrow).
MRI is an excellent tool for the diagnosis of extra-axial collections associated with meningitis, which can be sterile (effusion/hygroma) or infected (empyema). These complications are more common in pneumococcal meningitis affecting children under 2 years of age. Effusion/hygroma tends to be bilateral, large, and located in the frontal or temporal regions (▶ Fig. 8.2). On the other hand, empyemas can be located in the subdural or epidural spaces and are usually hyperintense to CSF on precontrast T1-weighted images, hyperintense on T2-weighted images, and show peripheral contrast enhancement and hyperintensity on DWI with reduced diffusion coefficients (▶ Fig. 8.3).1
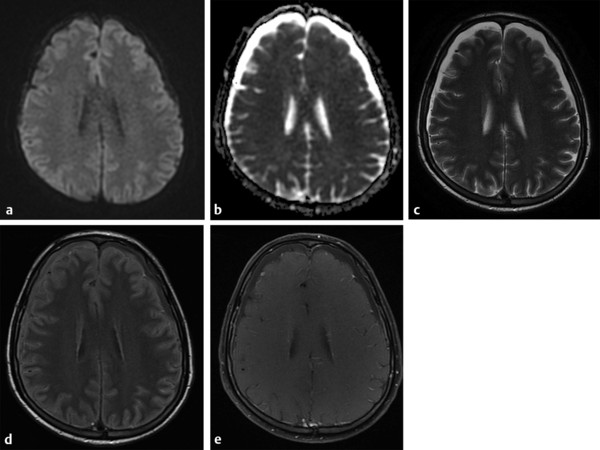
Fig. 8.2 Subdural effusion. Bilateral subdural effusion with signal intensity similar to cerebrospinal fluid (CSF), low signal on (a) axial diffusion weighted imaging and high signal on (b) apparent diffusion coefficient map, high signal on (c) T2, just more hyperintense to CSF on (d) fluid-attenuated inversion recovery, probably due to proteinaceous content, and no enhancement on postcontrast (e) T1-weighted image.
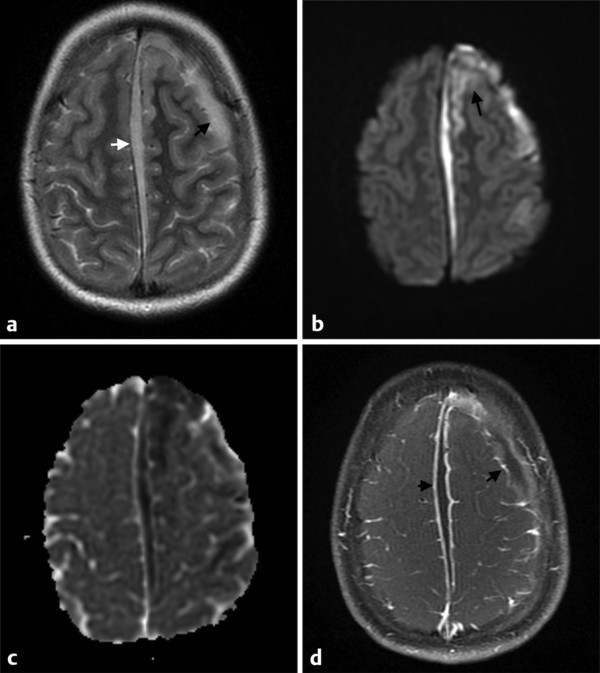
Fig. 8.3 This patient had a sinusitis and suffered a facial trauma, evolving to meningitis complicated with empyema, cerebritis and/or vasculitis and infarct in the left hemisphere. (a) The axial T2-weighted image shows a left frontal (black arrow) and parafalcine (white arrow) subdural collections, (b) which also show hyperintensity on diffusion weighted imaging (DWI) (c) and hypointensity on the apparent diffusion coefficient (ADC) map, typical of a subdural empyema. (b) Note also DWI hyperintensities in the left frontal gyri (arrow), which correlated with hypointensities on the ADC map and could be attributable to cerebritis or infarct. (d) The enhanced axial T1-weighted image shows contrast enhancement along the margins of the infected collections (arrows).
The diagnosis of pyogenic abscess is crucial for proper and prompt therapeutic management. The main etiologic agents associated with bacterial abscesses are Staphylococcus aureus and Streptococcus species.
Classically the evolution of brain abscess consists of four stages: early cerebritis, late cerebritis, early capsule formation, and late capsule formation. Regardless of the etiologic agent the MRI findings of the brain abscess are very similar.3 The first phase of cerebritis is characterized by vascular congestion, petechial hemorrhages, and edema, causing a poorly demarcated parenchymal softening. The MRI findings are nonspecific, with hyperintensity on T2-weighted images and little or no contrast enhancement. The capsular stage may begin approximately during the second week and can last for months, being characterized by a central zone of necrosis encircled by a collagen capsule. The abscess capsule can present as a smooth or lobulated hyperintense rim on T1-weighted and a hypointense rim on T2-weighted images with smooth and thin contrast enhancement. Because abscesses are ring-enhancing lesions many other differential diagnoses should be included, especially necrotic tumor. On DWI an abscess usually presents as hyperintensity with reduced apparent diffusion coefficients (ADCs) within the center of the abscess. In ring-enhancing lesions, if the ADC is low, the accuracy of the diagnosis of a capsular phase abscess versus necrotic tumor is high, and it is even higher when a complete T2-hypointense rim is present (▶ Fig. 8.4).3 The reduced ADC that occurs in the center of the lesion is attributable to pus that contains microorganisms, macromolecules, debris, proteins, amino acids, and inflammatory cells that bind, obstruct, and prevent the normal random motion of water.4 Even though low ADC in the center of a ring enhancing lesion is a sign of high accuracy for the diagnosis of bacterial abscess, some fungal abscesses and even necrotic tumors (especially metastases) can occasionally present restricted diffusion in its central portion, and, also, some bacterial abscesses may not have restricted diffusion. Brain abscess has a higher ADC (less restricted) after treatment than before treatment; thus DWI is a tool to evaluate treatment response (▶ Fig. 8.5 and ▶ Fig. 8.6). Relapse of a brain abscess decreases ADC values again.6
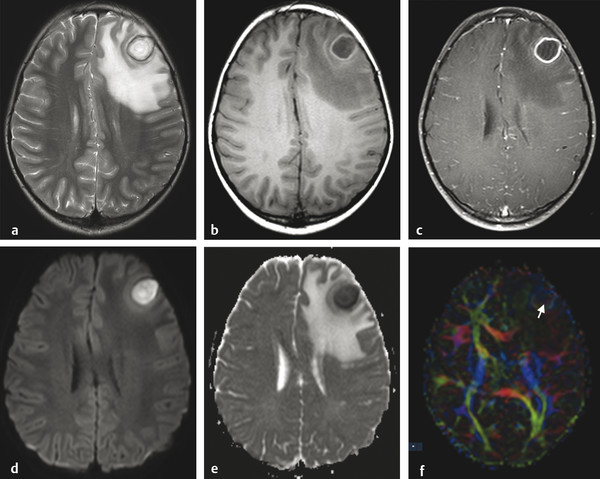
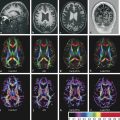
Fig. 8.4 Brain abscess and extensive edema within adjacent parenchyma. The abscess has a core of high signal intensity on the axial T2-weighted image (a), low signal on T1-weighted image pre-(b) and postgadolinium (c) and restricted diffusion with hyperintensity on the axial diffusion weighted imaging (d) and hypointensity on the apparent diffusion coefficient (ADC) map (e), whereas the adjacent edema has a facilitated diffusion (hyperintensity on the ADC map). There is a halo of hypointensity on the T2-weighted image (a) and hyperintensity on the T1-weighted image (b), with regular peripheral enhancement (c). The diffusion tensor imaging (f) usually shows a high fractional anisotropy value compared to other cystic lesions, and the color coded map may show an internal heterogeneous pattern (arrow), suggesting some organization that is typical of pus.
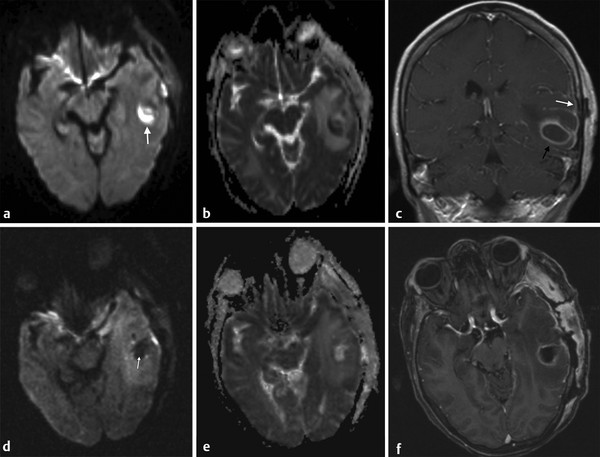
Fig. 8.5 Abscess (a) Axial diffusion weighted imaging (DWI) shows a hyperintense lesion in the left temporal lobe (arrow). (b) The apparent diffusion coefficient (ADC) of the lesion is reduced. (c) The coronal contrast-enhanced T1-weighted image shows peripheral enhancement of the lesion (black arrow) associated with meningeal involvement (white arrow). The diagnosis was a bacterial abscess. (d) After surgical drainage note reduction of the lesion size, with hypointensity of its core on DWI (arrow), (e) increased ADC, and persistent enhancement of the borders of the surgical cavity and adjacent meninges on (f) enhanced axial T1-weighted images. The lack of high DWI signal indicates that all pus has been drained.
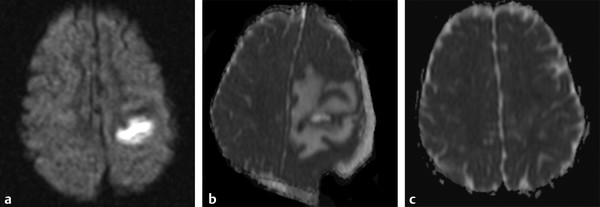
Fig. 8.6 Improvement of abscess postsurgery. (a) Axial diffusion weighted imaging shows a hyperintense lesion in the left hemisphere. (b) Three days following surgical drainage an axial apparent diffusion coefficient (ADC) map shows reduction of the lesion volume, increased peripheral edema, and an extra-axial postoperative collection. (c) One-month follow-up axial ADC map shows almost complete resolution of the lesion.
Gupta et al,7,8 using DTI, found increased fractional anisotropy (FA) in the cavity of the bacterial abscess (▶ Fig. 8.4f), which had a positive correlation with neuroinflammatory molecules, suggesting that FA elevation reflects upregulation of various adhesion molecules in brain abscess inflammation. Nath et al6 showed in 20 patients with abscesses that the FA in the cavities decreased following a successful 4 week treatment period.
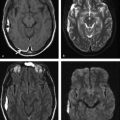
Ventriculitis is a rare intracranial infection, associated with abscess rupture into the ventricular system, extension of basal cistern meningitis to the ventricles, and ventricular empyema extension, or it may be iatrogenic. It is a potential fatal CNS infection. The ventricles are enlarged on MRI scans; the ependyma shows hyperintensity on fluid attenuated inversion recovery (FLAIR) and T2-weighted images, and there is contrast enhancement of the ventricular lining. Within the ventricles debris/suppurative content can sometimes be seen as -fluid levels, with sediments deposited in the depent parts. The debris/suppurative content presents hyperintensity on FLAIR images and on DWI with reduced ADC (▶ Fig. 8.7).9
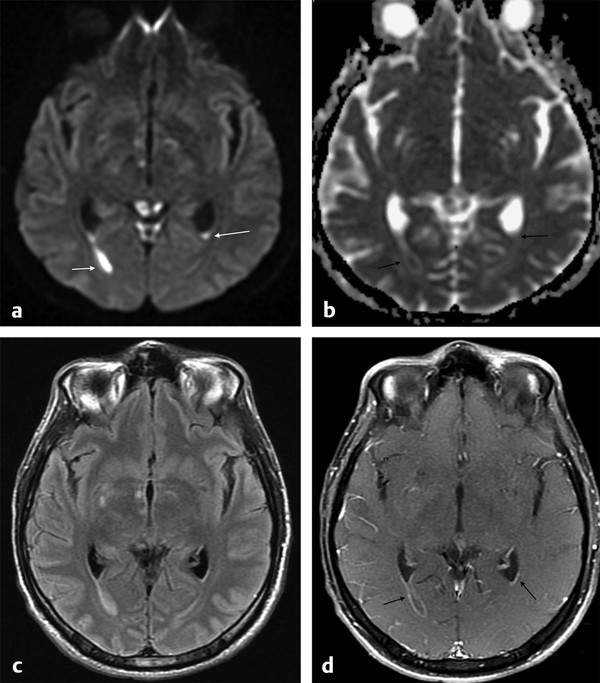
Fig. 8.7 Patient with pneumococcal meningitis and ventriculitis with restricted diffusion in the lateral ventricles, which can be seen as (a) hyperintense fluid levels (arrows) on diffusion weighted imaging and (b) hypointensity (arrows) on the apparent diffusion coefficient map. (c) The fluid-attenuated inversion recovery image shows hyperintensity in those locations, and (d) there is subependymal enhancement (arrows) on the enhanced T1-weighted image.
Other complications associated with meningitis are vascular, such as acute infarcts and venous thrombosis (▶ Fig. 8.8). DWI is a helpful diagnostic tool for the diagnosis of acute arterial infarcts but is less useful in venous infarcts, which may present as a variety of findings.
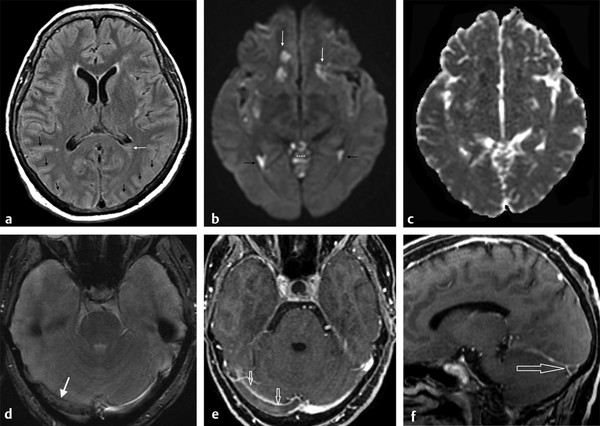
Fig. 8.8 Venous thrombosis due to meningitis. (a) Axial fluid-attenuated inversion recovery showing subtle hyperintensities in the sulci (black arrows) and in the posterior horn of the left lateral ventricle (white arrow). (b) The diffusion weighted imaging highlights the abnormalities in the lateral ventricles (ventriculitis) (black arrows), the meningitis in the cerebellar sulci (asterisks) and in the sylvian fissures, and also shows areas of restricted diffusion in subcortical areas (white arrows), also dark on apparent diffusion coefficient map (c), due to infarcts. (d) The T2 gradient-recalled echo shows hypointensity in the right transverse venous sinus (white arrow) that presents as a filling defect (open arrows) on the enhanced (e) axial and (f) sagittal images due to venous thrombosis.
In intracranial bacterial infections, neurotuberculosis (neurotb) must be emphasized because it has increased prevalence in immunocompromised patients, such as those with acquired immunodeficiency syndrome (AIDS) and organ transplants and those from endemic regions. The main presentation of neurotb is meningitis (up to 95% of cases) affecting mainly the basal meninges. Brain parenchyma involvement can be seen as a complication of meningeal involvement and includes infarcts or abscess/granuloma.10 DWI is useful for the diagnosis of acute ischemic infarcts, differentiating acute from subacute or chronic infarcts, and detecting vasculitis.11 Tuberculomas can present a T2-hypointense or T2-hyperintense core, and usually these lesions present with DWI hypointensity and ADC map hyperintensity (no restriction of diffusion) (▶ Fig. 8.9). However, DWI hyperintensity and ADC map hypointensity have also been described for tuberculomas.12 Both tuberculomas and tuberculous abscess can present as ring enhancement, central hyperintensity, or hypointensity on DWI, with or without reduced ADC, making differentiation between solid and necrotic caseation difficult on conventional MRI sequences and DWI (▶ Fig. 8.10).13 ▶ Table 8.1 summarizes the main DWI findings on bacterial infections.
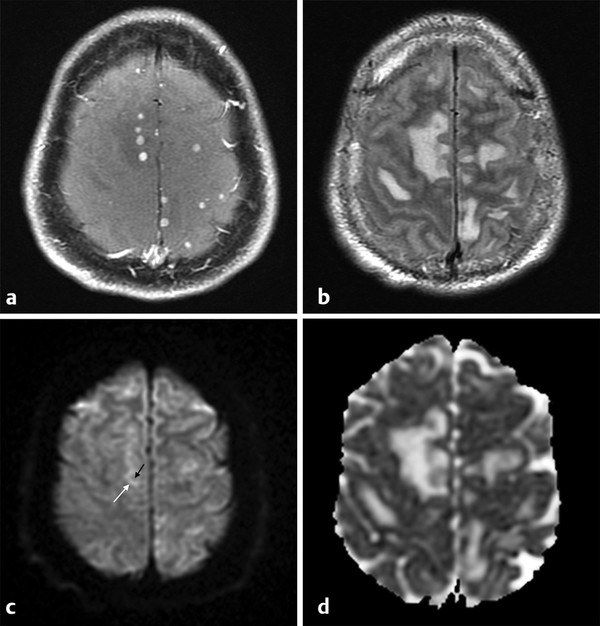
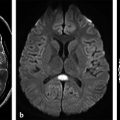
Fig. 8.9 Miliary tuberculosis. (a) Patient with multiple small contrast-enhancing nodules on T1-weighted image, (b) and edema on fluid-attenuated inversion recovery images. (c) The diffusion weighted imaging shows mild hyperintensity in the periphery (white arrow), and hypointensity in the core of the lesions (black arrow), (d) with no restricted diffusion on a corresponding apparent diffusion coefficient map.
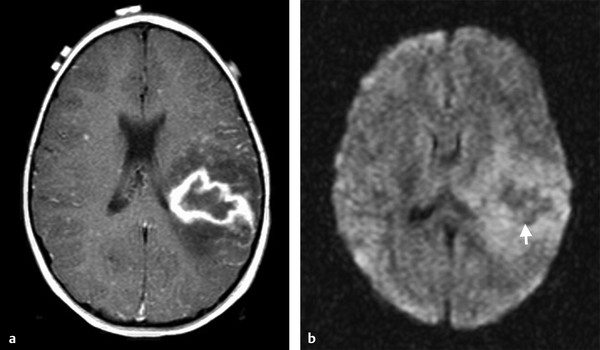
Fig. 8.10 Patient with tuberculosis. (a) Enhanced axial T1-weighted image shows a lesion with peripheral enhancement and perilesional edema. (b) Axial diffusion weighted imaging shows hypointensity on its central portion (arrow).
Lesions | DWI findings |
Meningitis | normal or CSF hyperintensity on DWI with no ADC restriction compared to parenchyma, but restricted compared to CSF |
Effusion/hygroma | Isointense or slightly hyperintense to CSF on DWI, no restricted diffusion on ADC maps |
Empyema | Hyperintense to CSF with low ADC |
Brain abscess | Hyperintense core on DWI with low ADC |
Successfully treated abscess | Increase ADC compared to untreated abscess |
Ventriculitis | Hyperintense intraventricular content on DWI with restricted ADC |
Abbreviations: ADC, apparent diffusion coefficient; CSF, cerebrospinal fluid; DWI, diffusion weighted image. | |
Viral Infectious
The diffuse and generalized infection of the brain known as encephalitis is most often the result of viruses. CNS viral infection can present as meningitis, encephalitis, and chronic encephalopathy.
The most common viral CNS infection is herpes encephalitis type 1, or oral herpesvirus, that carries a significant morbidity and mortality. The histopathology on herpes encephalitis is a fulminating necrotizing and, many times, hemorrhagic meningoencephalitis, predominantly in the temporal lobes, basal frontal lobes, insula, and cingulate gyri.14 The diagnosis of herpes encephalitis is made by polymerase chain reaction (PCR) techniques in the CSF, but this test can be negative within the first 72 hours of the disease. In such cases, DWI may play an important role in the detection of the characteristic lesions because early treatment is crucial. DWI shows hyperintensity in the affected regions with areas of decreased ADC, suggesting cytotoxic or excitotoxic edema (▶ Fig. 8.11). T2-weighted and FLAIR images show unilateral or bilateral hyperintense lesions in the temporal lobes, basal frontal lobes, insula, and/or cingulate gyri, with gyral edema, meningeal or cortical enhancement, and sometimes hemorrhagic foci. DWI shows the same abnormalities, but these are detected earlier and are easier to be identified than on other MRI sequences. Furthermore, DWI shows more extensive brain involvement than T1- or T2-weighted images, providing better mapping of the degree of brain involved.
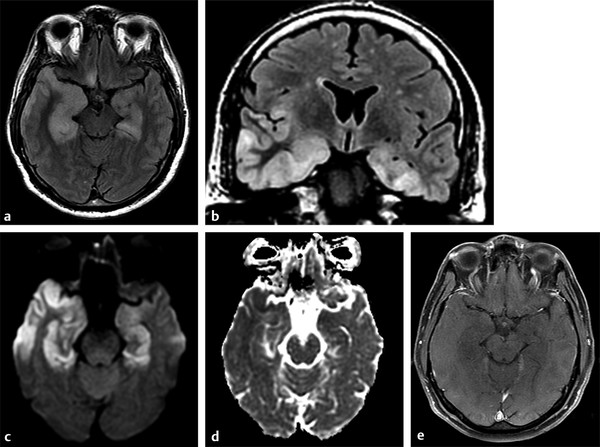
Fig. 8.11 Patient with herpes encephalitis. (a) Studies show bilateral temporal corticosubcortical involvement characterized by hyperintensity on axial and (b) coronal fluid-attenuated inversion recovery images, extending to frontal and insular areas. (c) There is hyperintensity on diffusion weighted imaging, (d) hypointensity on apparent diffusion coefficient map, and (e) no enhancement on enhanced T1-weighted image.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree