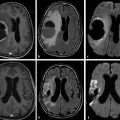
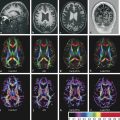
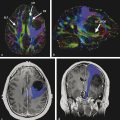
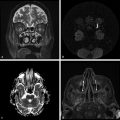
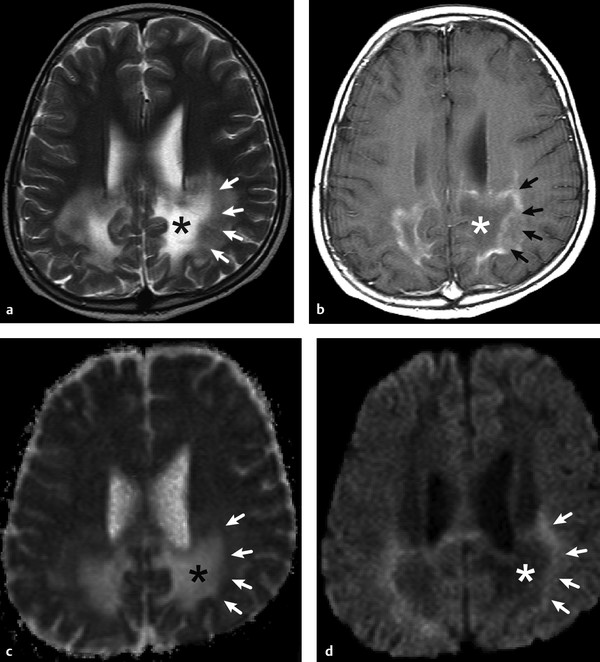
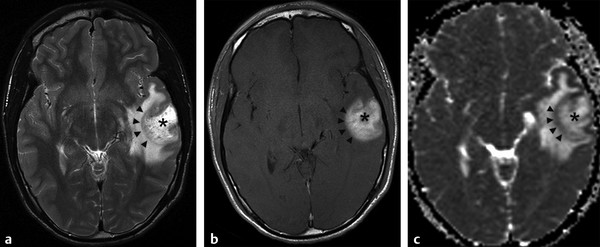
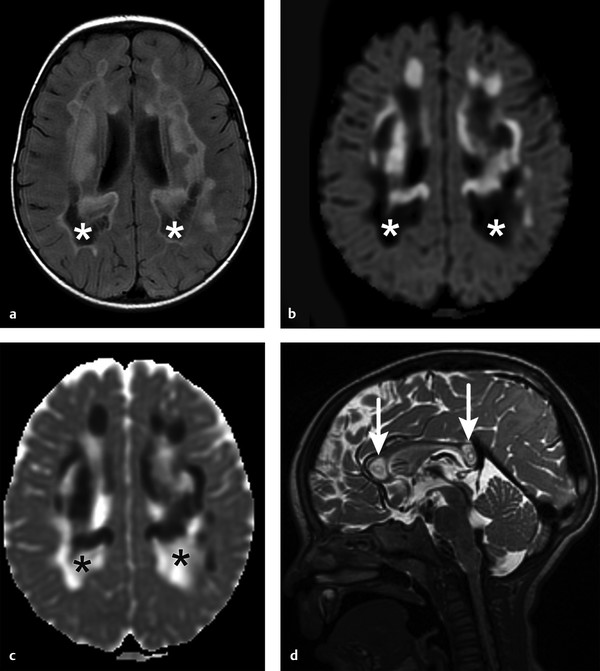
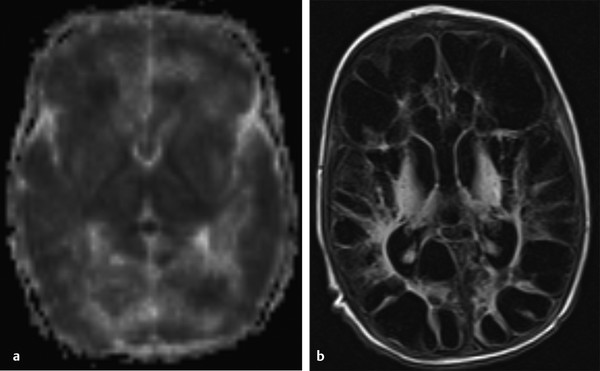
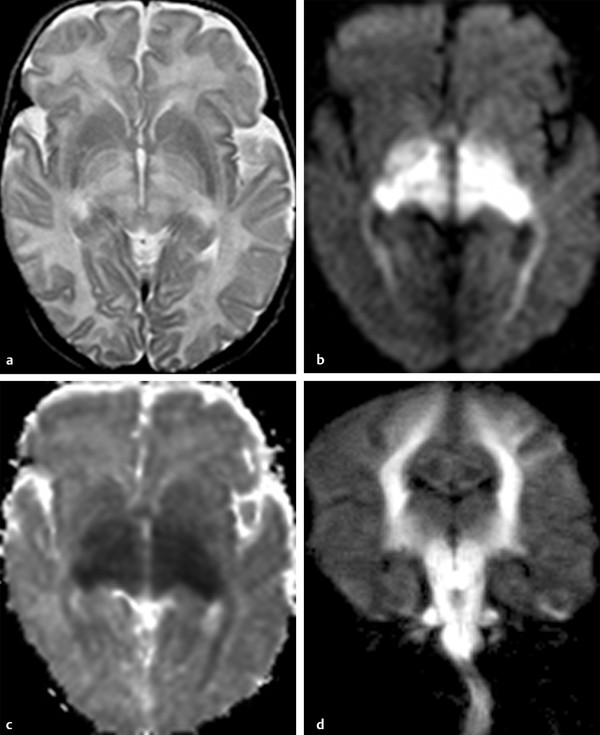
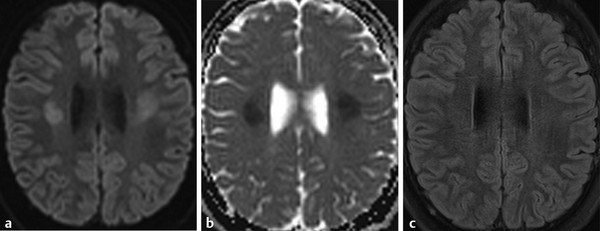
Physics In diffusion imaging, directional diffusion gradients are applied to a T2-weighted (spin-echo) echo-planar imaging (EPI) sequence before and after a 180-degree refocusing pulse. Water protons moving freely through these gradients acquire random spins and are thus dephased, causing signal loss in voxels where water motion (diffusion) is significant. Conversely, stationary or slowly moving protons generate increased signal on diffusion weighted images (DWIs). The apparent diffusion coefficient (ADC) may be calculated from images acquired without and with a diffusion gradient and allows quantitation of the displacement of water molecules over time (mm2/s) in a particular voxel.1,2 In tissues, water mobility may be restricted by cell membranes, macromolecules, or decreased extracellular spaces. In such cases, the signal on DWI is increased, and the ADC is decreased. For example, tissues with high cell density, such as small, round blue cell tumors like medulloblastoma and lymphoma, have decreased or “restricted” water diffusion and therefore appear bright on DWI. Hypercellularity due to a regional influx of cells, such as macrophages or glia, may also cause restricted diffusion (decreased ADC) and high signal on DWI. Cellular swelling due to cytotoxic edema, as in cerebral ischemia, also results in restricted diffusion due to resultant decreases in extracellular freely mobile water. Shift of water into the myelin sheath from the extracellular space, as occurs in intramyelinic edema or myelin vacuolation, may cause restricted diffusion in a similar or related fashion.3 This is in contrast to vasogenic edema, in which there is an accumulation of extracellular water due to blood–brain barrier (BBB) breakdown or osmotic shifts, causing increased ADC.4 Though all types of white matter edema may appear bright on T2-weighted imaging, ADC is increased in vasogenic edema (peritumoral edema, infection/inflammation), but decreased in cytotoxic edema (affecting oligodendrocytes and astrocytes) or intramyelinic edema, permitting differentiation. However, different types of edema may coexist (see “maple syrup urine disease” later in this chapter) and even shift during the course of disease. By providing insight into the dominant edema type, diffusion imaging may help characterize dynamic white matter disease processes. Although diffusion is apparently equal in all directions (isotropic) in gray matter, in white matter it is greatest parallel to the fiber bundles (“tracts”) and restricted perpendicular to the tracts, a property known as anisotropy. This is exploited in diffusion tensor imaging (DTI) and fiber tracking, and may be quantified as fractional anisotropy (FA), a measure of the propensity of water to diffuse in a single direction (e.g., along a white matter tract). An FA of 0 indicates isotropic diffusion, and the maximal FA of 1 indicates perfectly linear, anisotropic diffusion along the primary (longitudinal) eigenvector, which indicates axonal orientation. The mean of the second and third eigenvectors, which are perpendicular to the axon, is known as radial diffusivity and can provide further quantitation of myelin integrity. These metrics offer exciting, noninvasive, and reproducible approaches to qualitative and quantitative evaluation of white matter diseases. In the central nervous system (CNS), the lamellar myelin sheath is formed by the wrapping of specialized oligodendrocyte membrane layers around intact axons, a process that is driven by neuronal electrical activity, coordinated activation of myelin protein genes, and growth and trophic factors produced by neurons and astrocytes. Disorders of any of these components may primarily or secondarily affect myelin to a variable degree. Hypomyelination describes early and permanent arrest in myelination (Pelizaeus–Merzbacher disease, trichothiodystrophy), as opposed to delayed myelination, in which myelin is quantitatively inappropriate for the age of the patient, typically infants. When myelination progresses but is abnormal due to defective, absent, or accumulated myelin components, it is termed dysmyelination.5 Demyelination, the loss of qualitatively normal myelin, may be secondary (due to neuronal or axonal loss, as in trauma) or primary (due to abnormalities of myelin or oligodendrocytes). Primary demyelinating disorders may be heritable (metachromatic leukodystrophy, X-linked adrenoleukodystrophy), or acquired (infection, inflammation, immune disorders, toxins, ischemia). Because these classic categories are descriptive rather than histopathologic, they may overlap and coexist. Dysmyelination predisposes to demyelination; hypomyelination often accompanies or may mimic delayed myelination. Similarly, though MRI-based patterns of pediatric white matter diseases may occasionally provide a high degree of diagnostic specificity,6 conventional imaging abnormalities are not specific to underlying histopathologic processes. T1 hypointensity may occur due to rarefaction of myelin (and tissue matrix) or edema; T2 hyperintensity may be due to cytotoxic, intramyelinic, or vasogenic edema, or increased white matter water content due to decreased myelin. The precise pathomechanisms for many pediatric white matter disorders remain unknown, limited by the rarity of the disorders and limited availability of pathological material at various stages of disease. The ability of diffusion imaging to differentiate white matter pathologies contributes to our growing understanding of the pathological mechanisms underlying these disorders, and becomes stronger as more radiological–pathological–molecular biological correlation becomes available. Because patterns of diffusion abnormalities differ based on underlying mechanism, diffusion imaging may contribute to the imaging semiology and improve the specificity of traditional pattern-based diagnosis. Because many white matter diseases are characterized by diffusion abnormalities in their early stages, which subsequently progress to nonspecific increases in water diffusivity and decreased anisotropy due to demyelination and destruction of the white matter matrix, DWI/DTI may also be helpful in assessing the stage and rate of progression of these diseases, particularly in the early stages of disease. The overall number of pediatric white matter disorders is already significant, and new entities are identified almost every year; the precise mechanisms of white matter damage and changes on brain magnetic resonance imaging (MRI) have yet to be elucidated in most. We therefore focus on disorders for which pathomechanisms and how they relate to diffusion imaging findings are understood (with a reasonable degree of confidence) to illustrate how DWI/DTI can contribute to pathological understanding and diagnosis in these and other pediatric white matter disorders. Accumulation of microglia and myelin-laden macrophages (hypercellularity) and cytotoxic edema of oligodendrocytes due to proinflammatory cascades and/or metabolic stress results in restricted diffusion in areas of active demyelination.7,8 Once most or all myelin is lost, these areas demonstrate increased diffusivity and decreased FA in the “burned-out” phase.9 In X-linked adrenoleukodystrophy (X-ALD), a single-enzyme defect in peroxisomal function results in accumulation of very long chain fatty acids (VLCFAs), which disrupt membranes and are toxic to oligodendrocytes and, to a lesser degree, astrocytes. Early demyelination becomes suddenly inflammatory in 90%, with rapid progression.10 Progression is typically centrifugal and posterior to anterior.11 Diffusion is relatively decreased in the inflammatory “leading edge” (▶ Fig. 10.1), which pathologically consists of perivascular inflammatory infiltrates, lipid-laden macrophages, and reactive astrocytes. In the central demyelinated zone, where myelinated axons and oligodendrocytes are essentially absent, diffusivity is increased and FA is decreased.12 Fig. 10.1 X-Linked adrenoleukodystrophy. (a) Axial T2-weighted image, (b) enhanced T1-weighted image, (c) diffusion weighted image, and (d) apparent diffusion coefficient map. Diffusion is relatively decreased in the peripheral, enhancing inflammatory zone (arrows) surrounding the central demyelinated zone (*), in which water diffusivity is increased. Multiple sclerosis is a clinically and pathologically heterogeneous chronic inflammatory demyelinating disorder. Active inflammatory lesions show variable perivascular inflammation, T lymphocytes, and lipid-laden macrophages, whereas chronic lesions are hypocellular and show variable remyelination.13 Though most white matter lesions demonstrate increased diffusion, restricted diffusion has been reported in ~ 30% of acute lesions, usually the tumefactive type, likely reflecting the heterogeneity of underlying pathological mechanisms.7,8 Axons are relatively preserved. When present in the acute phase, restricted diffusion is most common at the periphery of the lesion, may precede T2 signal abnormality and enhancement, and correlates with attack severity.9 FA is lower in enhancing than in nonenhancing lesions.14 Acute disseminated encephalomyelitis is characterized pathologically by perivenular demyelination and inflammation, occurring after viral illness or infection.13 Monophasic white matter lesions tend to be large and bilaterally asymmetric, with increased diffusion in the core and variable, often decreased, ADC at the periphery in the acute phase (▶ Fig. 10.2).15 Fig. 10.2 Tumefactive demyelination. (a) Axial T2-weighted image, (b) enhanced T1-weighted image, and (c) apparent diffusion coefficient map show three distinct zones: an enhancing core with increased water diffusion (*) surrounded by a rim of enhancing restricted diffusion corresponding to active inflammation (arrowheads), and peripheral vasogenic edema. (Courtesy of Dr. W. K. Chong, Ormond Street Hospital for Children, NHS Trust, London, UK.) In mitochondrial disorders, the mitochondria are unable to meet the metabolic requirements of the cell (metabolic imbalance), resulting in cytotoxic edema in the acute phase.16 Though most mitochondrial disorders involve deep gray matter with or without some white matter involvement, some selectively affect white matter. Isolated deficiencies of respiratory chain complexes may result in a characteristic cavitating leukoencephalopathy, with a distinct pattern of strikingly restricted diffusion throughout the white matter (including the corpus callosum) during the acute phase, with the leading edge apparently moving centrifugally, which may coexist with increased diffusivity and loss of FA in white matter cavitations in the central burned-out areas (▶ Fig. 10.3).17 Fig. 10.3 Complex I (respiratory chain) deficiency in a 12-month-old infant with seizures and rapid neurological deterioration. (a) Axial fluid-attenuated inversion recovery images show extensive white matter abnormality with sparing of the subcortical white matter and presence of central cavitations (*). (b) Diffusion weighted image and (c) apparent diffusion coefficient map show strikingly restricted diffusion in affected white matter and increased diffusivity in cavitations. (d) Involvement of the corpus callosum (arrows) is evident on sagittal T2-weighted imaging. (Courtesy of Drs. Fred Laningham and Natalie Hauser, Children’s Hospital of Central California, Madera, CA, USA.) In certain instances, severe perinatal hypoxia in term infants may result in acute global neonatal ischemia with diffuse supratentorial cytotoxic edema due to cerebral energy depletion, with restricted diffusion predominantly involving subcortical white matter (▶ Fig. 10.4a), progressing to widespread cavitation (▶ Fig. 10.4b) with increased diffusivity. The brainstem and posterior fossa are generally spared.6,18 Neonatal herpes infection and isolated sulfite oxidase deficiency are in the differential diagnosis for this appearance.19 Fig. 10.4 Severe global hypoxic injury in a 4-day-old infant delivered at 38 weeks with vasa previa. (a) Diffusely abnormal diffusion on apparent diffusion coefficient map at 4 days, which (b) progressed to diffuse cerebral cavitation and atrophy on fluid-attenuated inversion recovery magnetic resonance imaging by 26 days of life. In these disorders, rapid extracellular-to-intracellular or intramyelinic fluid shift results in decreased free water diffusion. In classic maple syrup urine disease, autosomal recessive deficiency in branched-chain ketoacid dehydrogenase (a mitochondrial enzyme complex) results in accumulation of branched-chain amino acids (especially L-leucine) and their keto-acids (especially α-ketoisocaproic acid (αKIC). Overabundant leucine competitively inhibits uptake of other amino acids into cells, inhibiting protein synthesis; myelin synthesis is impaired leading to dysmyelination. At the same time, αKIC inhibits adenosine triphosphate (ATP) synthesis, disrupting sodium–potassium–ATP (NA/K ATPase) function. Because myelination in the newborn and young infant is extremely active, hence energy dependent, cytotoxic and intramyelinic edema (and restricted diffusion) develop exclusively in myelinated areas, superimposed on a background of diffuse vasogenic edema as a sign of global neurotoxicity (▶ Fig. 10.5).20,21 Fig. 10.5 Maple syrup urine disease. Diffuse vasogenic edema on (a) T2-weighted image, with restricted diffusion in myelinated white matter on (b) diffusion weighted image (DWI) and (c) apparent diffusion coefficient map. (d) Coronal DWI shows involvement of myelinated white matter not only intracranially, but also in the spinal cord. Methotrexate (MTX) inhibits dihydrofolate reductase, resulting in adenosine release, which dilates cerebral blood vessels and slows presynaptic neurotransmitter release.22 Because symptoms improve rapidly with aminophylline, a competitive adenosine antagonist, MTX neurotoxicity is favored to be mediated by the actions of adenosine in the CNS via unknown mechanisms, potentially via oxidative stress.22,23 Homocysteine may be increased or normal.24 Ten to 14 days after MTX administration, and within hours after the appearance of clinical signs and symptoms, restricted diffusion develops in the centrum semiovale of affected patients (▶ Fig. 10.6a,b). This is unaccompanied by T2 or fluid-attenuated inversion recovery (FLAIR) evidence of vasogenic edema in the early stage (▶ Fig. 10.6c), consistent with a “pure” cytotoxic and/or intramyelinic edema. Therefore DWI is critical for early, confident diagnosis of MTX neurotoxicity. This restricted diffusion subsequently subsides, but in some cases may progress to loss of tissue matrix, with increased diffusivity and T2 signal in the primarily involved areas.24 Fig. 10.6 Acute methotrexate toxicity. Restricted diffusion on (a) diffusion weighted image and (b) apparent diffusion coefficient map, unaccompanied by (c) fluid-attenuated inversion recovery (FLAIR) signal abnormality, consistent with cytotoxic/intramyelinic edema.
10.2 Clinical Applications
10.2.1 Restricted Diffusion: Active Inflammatory Demyelination
Inherited: X-linked Adrenoleukodystrophy
Acquired: Multiple Sclerosis, Acute Disseminated Encephalomyelitis
10.2.2 Restricted Diffusion: Diffuse Cytotoxic/Intramyelinic Edema with White Matter Cavitation
Inherited: Mitochondrial Respiratory Chain Deficiency
Acquired: Global Neonatal Hypoxic Injury
10.2.3 Restricted Diffusion, Regional: Neurotoxicity
Inherited: Maple Syrup Urine Disease (Classic Type)
Acquired: Methotrexate Toxicity
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree