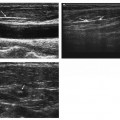
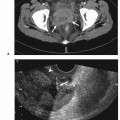
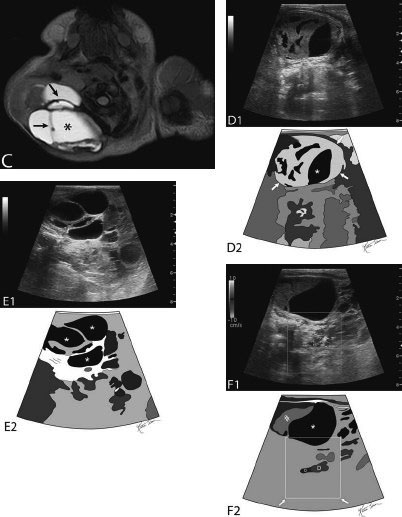
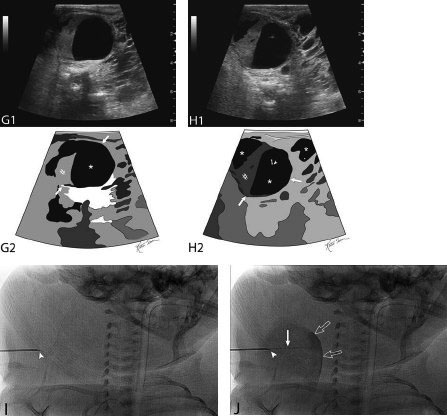
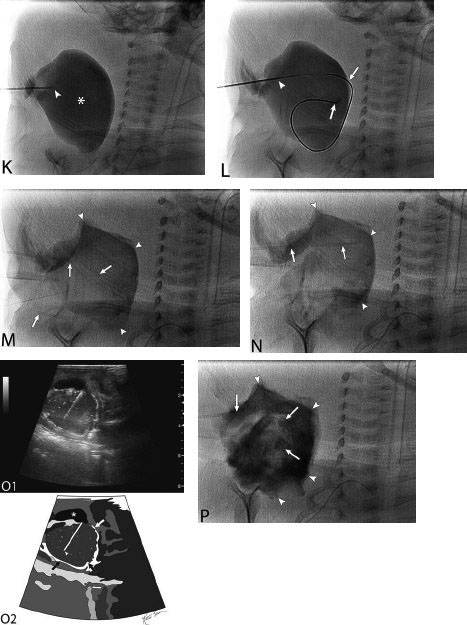
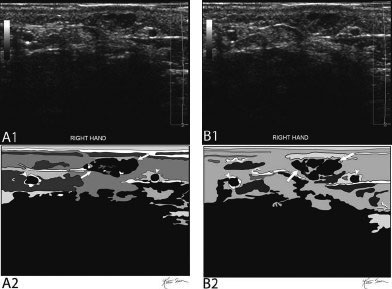
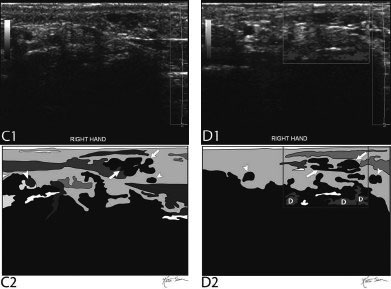
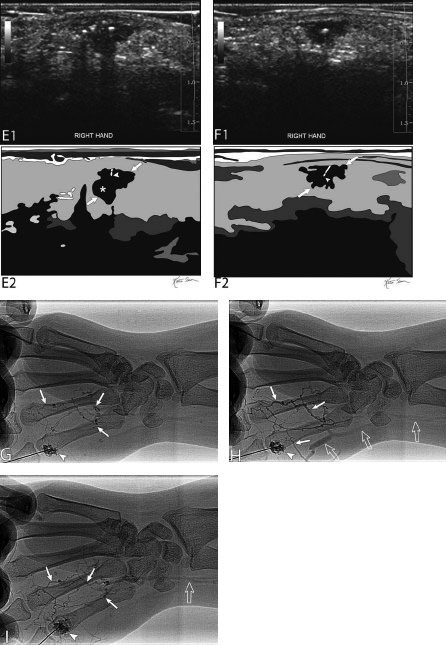
21 Direct Percutaneous Sclerosis of Vascular Malformation Vascular malformations are classified into hemangiomas and vascular malformations proper. With respect to ultrasound-guided procedures, vascular malformations are classified here into high-flow and low-flow vascular malformations. Numerous methods, with technical variants within each method, for the management of vascular malformations have been described. However, the focus of this chapter is ultrasound-guided direct percutaneous sclerosis of vascular malformations. Transcatheter/endoluminal techniques in managing vascular malformations are not addressed in this chapter. Here the indications for treating arteriovenous malformation (AVM) in general are provided, as well as guidelines for the use of direct percutaneous sclerosis techniques over transcatheter/endoluminal techniques. The ideal features of particular AVMs more amenable to direct percutaneous ultrasound-guided needle access and sclerotherapy are described. My preference is to treat vascular malformations via an endoluminal approach when possible rather than a direct percutaneous approach. The ultrasound-guided direct percutaneous approach is reserved for vascular malformations that are poorly accessible by endoluminal means. Fig. 21.1 Lymphangioma (cystic hygroma): imaging and therapy. (A) Axial computed tomography (CT) image of the upper neck at the level of the mentum (M, mentum/mandible) of a neonate with Klippel-Trenaunay syndrome exhibiting multiple neck, axillary, and chest lymphangiomas. The low-density areas are the cystic lymphangiomas. Asterisks mark some of the clearly defined cysts. The lymphangiomas are seen dissecting deep around the carotid artery (arrowhead), which seems suspended in the lymphangioma. In this image, the lymphangioma is seen dissecting deep and crossing the midline (from right to left) posterior to the trachea (T) with an endotracheal tube in it and the esophagus (E) with an nasogastric tube in it and anterior to the vertebral (V) column. The left clavicular (C) head is incidentally seen in this axial image. (B) Axial CT image of the upper neck at a higher (more cephalad) level than in Fig. 21.1A (despite this, the clavicle, C, is seen in its shaft because the left shoulder girdle is raised due to left axillary lymphangiomas which are not seen in this image). Again, seen in the low-density areas are the cystic lymphangiomas. Asterisks mark the continuum of the clearly defined cysts marked in Fig. 21.1A. The lymphangiomas are seen dissecting deep around the carotid artery (arrowhead), which seems suspended in the lymphangioma. In this image, the lymphangioma is seen dissecting deep and crossing the midline (from right to left) posterior to the trachea (T) with an endotracheal tube in it and the esophagus (E) with an nasogastric tube in it and anterior to the vertebral (V) column. (C) Axial T2-weighted magnetic resonance (MR) image of the neck of the same neonate with Klippel-Trenaunay syndrome. Again seen are the cystic components of the lymphangiomas with their high T2-weighted signal intensity. Low signal intensity septa are seen (arrows) traversing the macrocytic part of the lymphangioma. An asterisk marks one of the larger simple cystic components of the lymphangioma. (D) Gray-scale ultrasound image (top) and schematic sketch (bottom) of the lymphangiomas of the right neck of the same neonate as in Figs 21.1A–21.1C. The complexity of the lymphangiomas (between arrows) now is more apparent that what was conveyed in the axial CT and MR images. An asterisk marks one of the larger cystic components of the lymphangioma. Around it is the sponge-like appearance of the soft tissue component of the malformation (arrows pointing to lymphangioma). (E) Gray-scale ultrasound image (top) and schematic sketch (bottom) of the same lymphangioma of the right neck as in Fig. 21.1D. More cystic compartments (*) with traversing septa are seen in this part of the lymphangioma. (F) Doppler image (top) (arrow pointing to Doppler box) and schematic sketch (bottom) of the same lymphangioma as in Figs. 21.1A–21.1E. The carotid vessels (D, Doppler) are seen deep to this particular cystic component of the lymphangioma. The asterisk and the # sign mark the cystic and soft tissue aspects of the visualized lymphangioma component, respectively. (G) Gray-scale ultrasound image (top) and schematic sketch (bottom) of the lymphangiomas of the right neck of the same neonate just prior to accessing the cyst for direct percutaneous sclerotherapy. The asterisk and the # sign mark the cystic and soft tissue aspects of the visualized lymphangioma (between arrows) component, respectively. (H) Gray-scale ultrasound image (top) and schematic sketch (bottom) of the lymphangiomas of the right neck after accessing a cystic locule with a 21-gauge needle (arrowhead). The asterisks and the # sign mark the cystic and soft tissue aspects of the visualized lymphangioma (between arrows) component, respectively. At this time, the procedure is converted from real-time ultrasound guidance to real-time fluoroscopic guidance. (I) Fluoroscopic spot image with the 21-gauge needle in the cystic component of the lymphangioma (arrowhead at needle tip). (J) Fluoroscopic spot image during contrast injection through the 21-gauge needle in the cystic component of the lymphangioma (arrowhead at needle tip). Notice the jet of contrast (solid arrow) as it traverses the cyst and hits the back wall (hollow arrows) of the cystic locule.(K) Fluoroscopic spot image after contrast injection through the 21-gauge needle in the cystic component (*) of the lymphangioma (arrowhead at needle tip). There is no communication with vital cavities or draining veins. It is safe to inject the sclerosant. The operators prefer to use a catheter or sheath and not a needle especially as a sclerosant such as doxicycline is used, which requires a long sclerosant dwell time 30–60 minutes prior to aspiration. (L) Fluoroscopic spot image with the 21-gauge needle in the cystic component of the lymphangioma (arrowhead at needle tip). A 0.018-inch wire has been passed through the needle and is coiling in the cystic locule (arrows). (M) Fluoroscopic spot image with a 5-French short micropuncture sheath in the collection (arrows). The contrast in the cyst has been partly aspirated prior to the doxycycline concoction administration. (N) Fluoroscopic spot image as the 5-French short micropuncture sheath in the collection is being pulled back (arrows). The operator is aspirating the contrast as the sheath is being pulled back, but not completely out (arrowheads). Blunt (sheath) access is to be maintained to administer the doxycycline mixture (300 mg of doxycycline powder reconstituted by 15 cc of normal saline and 5 cc of 1% lidocaine). (O) Gray-scale ultrasound image (top) and schematic sketch (bottom) of the lymphangiomas of the right neck after the doxycycline mixture (300 mg of doxycycline powder reconstituted by 15 cc of normal saline and 5 cc of 1% lidocaine) has been administered through the 5-French sheath (arrowhead at tip of 5-French sheath) into the target locule (arrows). Notice the isoechoic fluid mixture, which is more echogenic due to small bubbles suspended within the doxycycline. Superficial to the target locule is an untreated (not accessed) adjacent cyst (*). (P) Fluoroscopic spot image with the 5-French short micropuncture sheath in the collection (arrows). The doxycycline mixture (300 mg of doxycycline powder reconstituted by 15 cc of normal saline and 5 cc of 1% lidocaine) has been dwelling in the cyst (arrowheads) for 30–40 minutes. Fig. 21.2 Slow-flow venous malformation: ultrasound imaging and therapy. (A) Gray-scale ultrasound image of a superficial and small slow-flow venous malformation in the palm (hypothenar eminence) of a young woman’s hand (top) and schematic sketch of it (bottom). The arrows point to the localized hypoechoic tuft of vessels. Adjacent palmar arteries are marked with arrowheads. (B) Gray-scale ultrasound image of the same superficial slow-flow venous malformation in the palm (top) at an adjacent site and schematic sketch of it (bottom). The arrows point to the localized hypoechoic tuft of vessels. Adjacent palmar arteries are marked with arrowheads. (C) Gray-scale ultrasound image of the same superficial slow-flow venous malformation in the palm (top) at an adjacent site and schematic sketch of it (bottom). The arrows point to the localized hypoechoic tuft of vessels. Adjacent palmar arteries are marked with arrowheads. (D) Doppler image (top) and schematic sketch of it (bottom) of the same venous malformation (arrows) as in Figs. 21.2A–21.2C. Power Doppler ultrasound coloring (D) deep to the venous malformation is seen. However, power Doppler is not able to detect flow within the venous malformation. This is typical of slow-flow venous and lymphangiovenous malformation where blood flow is so slow that it is not picked up by color Doppler or even the more sensitive power Doppler. Adjacent palmar arteries are marked with arrowheads. (E) Gray-scale ultrasound image of the same superficial slow-flow venous malformation in the palm (top) at an adjacent site and schematic sketch of it (bottom). The venous malformation (* = tuft of abnormal veins) has been accessed using a 23-gauge butterfly needle (arrowhead at needle tip). The arrows point to the outer confines of the tuft of abnormal venous vessels. (F) Gray-scale ultrasound image of the same superficial slow-flow venous malformation in the palm (top) at an adjacent site and schematic sketch of it (bottom). The venous malformation has been accessed using a 23-gauge butterfly needle (arrowhead at needle tip). The arrows
Classification and Indication
Indications for Treating Vascular Malformations
Indications for Ultrasound-Guided Direct Percutaneous Vascular Malformation Sclerosis
Contraindications
Relative Contraindication
Absolute Contraindication
Preprocedural Evaluation
Evaluate Prior Cross-Sectional Imaging
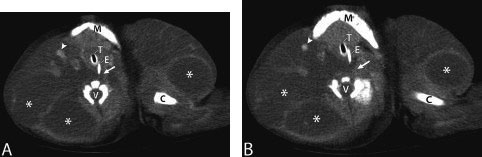
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree