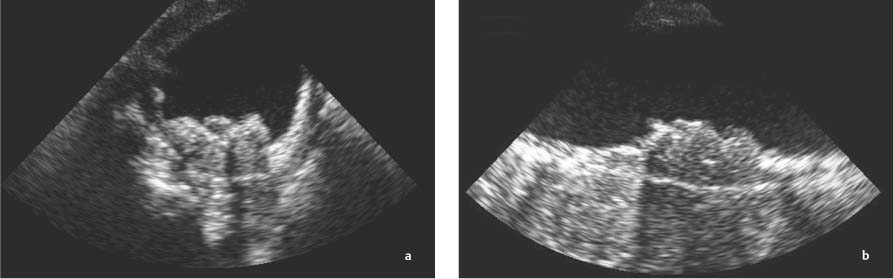
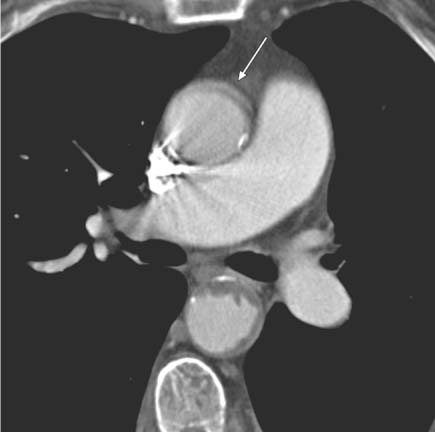
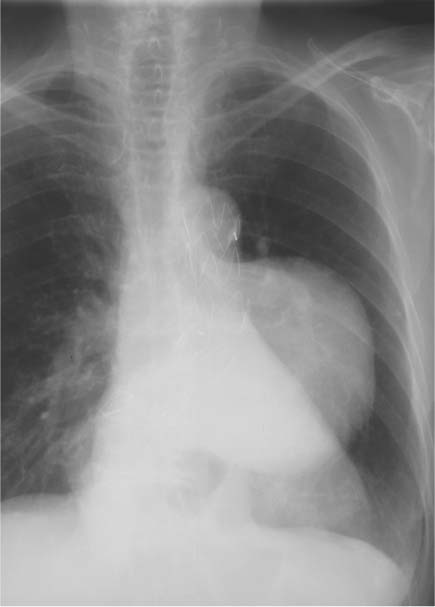
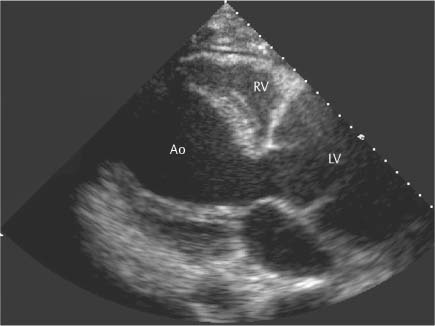
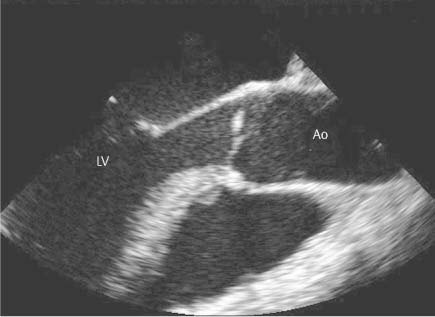
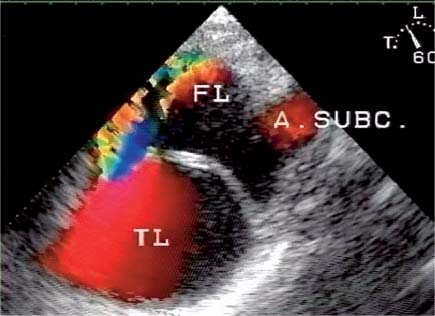
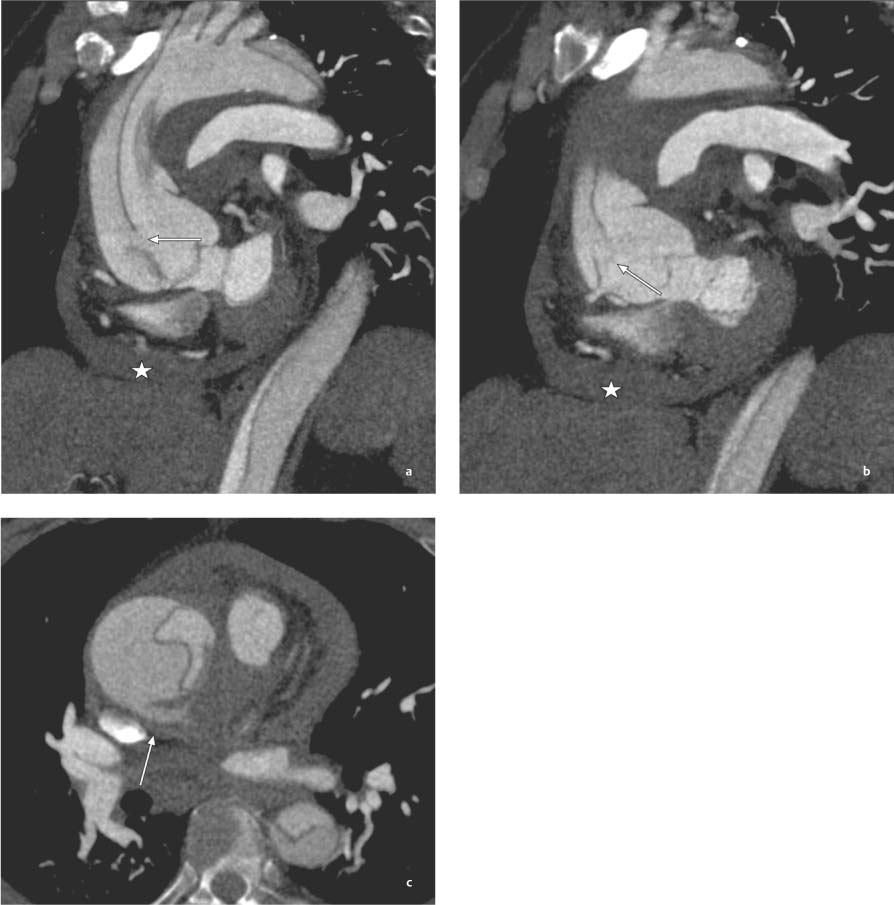
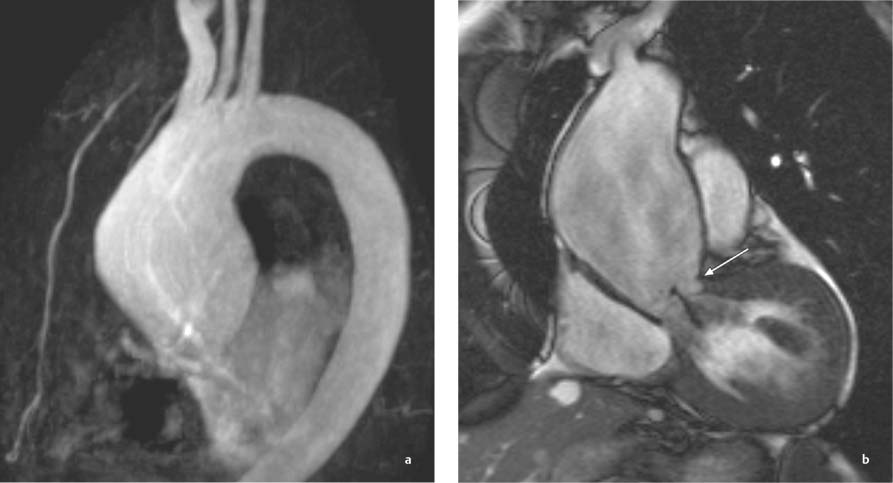
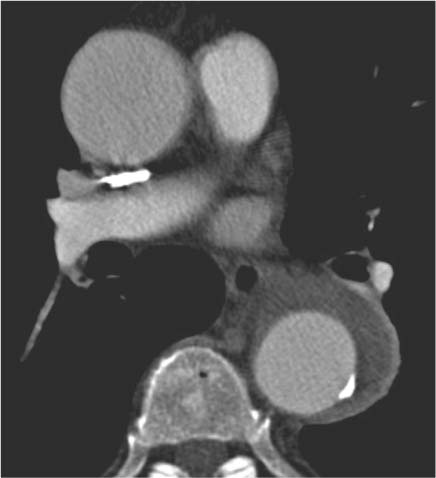
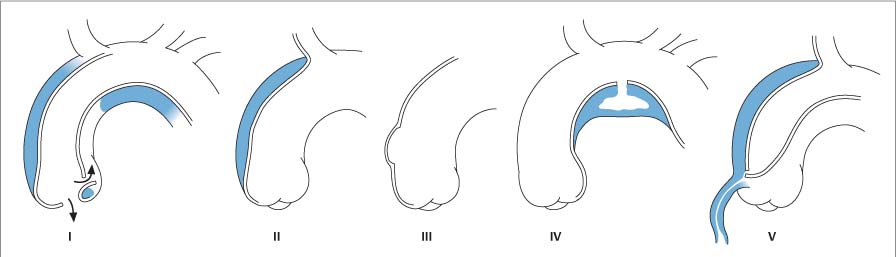
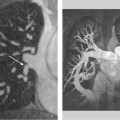
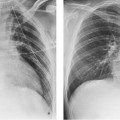
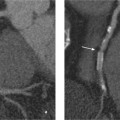
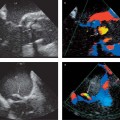
13 Diseases of the Thoracic Aorta Besides congenital anomalies, the most common diseases of the thoracic aorta are acquired, usually degenerative diseases that affect the aortic wall. Atherosclerotic wall changes, disorders of connective-tissue metabolism (e. g., in Marfan syndrome), or inflammatory infiltration of the vessel wall can lead to thickening of the aortic wall, aortic dilatation (ectasia), dissection, or aneurysm formation. Acute aortic syndrome often takes a fulminating course. This requires a rapid diagnostic evaluation to provide an immediate basis for making therapeutic decisions that will critically affect the prognosis. The aorta begins at the aortic valve and extends distally to the aortic bifurcation. The aorta is divided into four parts: • Ascending aorta • Aortic arch • Supradiaphragmatic descending thoracic aorta • Infradiaphragmatic descending abdominal aorta The ascending aorta originates in the supravalvular part of the left ventricle. The annulus of the ascending aorta bears the aortic valve. The initial portion of the ascending aorta, the aortic root, forms a bulbous expansion (the aortic bulb) that bears three dilatations corresponding to the cusps of the aortic valve. The left and right coronary sinuses (of Valsalva) give origin to the left and right coronary arteries, respectively, and are accompanied by a third, acoronary, sinus. The aortic bulb tapers at the sinotubular junction to become continuous with the ascending aorta. The aortic arch runs upward, backward, and to the left, passing over the right pulmonary artery and left main bronchus. The aortic arch gives off the supra-aortic arteries. This region should be closely scrutinized for any variants in the origins of the arch vessels. The descending thoracic aorta lies just to the left of the vertebral column and descends to the aortic hiatus of the diaphragm. The descending aorta gives off the bronchial arteries, spinal arteries, and smaller arterial branches. The descending aorta may show considerable elongation and tortuosity, especially in older individuals, but usually this does not cause clinical complaints. The descending aorta is smaller in diameter than the ascending aorta and tapers only slightly in its course. The part below the aortic hiatus is called the abdominal aorta. A right descending aorta in the absence of visceral transposition is a very rare developmental anomaly. It involves a mirror-image reversal of anatomy in which the brachiocephalic trunk supplies the left side, followed in turn by the right subclavian artery and right carotid artery. A very high percentage of patients have a coexisting cardiac anomaly, usually a tetralogy of Fallot or a truncus arteriosus. The most common clinical manifestation of a right descending aortic arch is dyspnea due to tracheal compression by the left subclavian artery, which passes behind the trachea and esophagus. In a double aortic arch, the ascending aorta is divided into two parts that run to the right and left of the esophagus and trachea. In most cases this division occurs just anterior to the trachea. The two limbs of the aortic arch pass around the trachea and reunite behind it and the esophagus, or rarely in front of the esophagus, to form a central or left-sided descending aorta. Hence this anomaly is also called the “vascular ring.” Normally, one of the aortic arches is small or rudimentary (Fig. 13.1). A double aortic limbs is not usually associated with other cardiovascular anomalies. In most cases, however, it produces early clinical manifestations because the ring around the trachea causes extrinsic tracheomalacia and dyspnea.1 A far posterior origin of the left subclavian artery may signify a coarctation or other aortic anomaly. Among the many possible variants in vessel origins, the aberrant right subclavian artery has the greatest clinical importance (prevalence of 0.5–1% in the general population). It arises behind and below the apex of the aortic arch and runs to the right, passing behind the trachea and esophagus. This vessel may cause dysphagia because of its retroesophageal course (Fig. 13.2). Congenital hypoplasia of the aortic arch, known also as Williams–Beuren syndrome, is very rare. Fig. 13.1 Aortic arch anomaly in a 45-year-old man with a right-sided aortic arch and rudimentary left aortic arch, a right descending aorta, and separate origins of all supra-aortic vessels (sequence: left common carotid artery, right common carotid artery, right subclavian artery, and left subclavian artery). Fig. 13.2 Aberrant right subclavian artery, which is the last vessel arising from the aortic arch. All the supra-aortic vessels arise separately from the aortic arch. Coarctation is the most common congenital anomaly of the thoracic aorta. It has an incidence of ~4.1 in 10 000 newborns and is present in ~7% of patients with congenital heart disease. Preductal and postductal forms are distinguished. The preductal form, which is symptomatic in the neonatal period, is associated with prestenotic hypoplasia of the transverse aortic arch in ~80% of cases (Fig. 13.3). Only 10–16% of patients with the postductal form become symptomatic during infancy. As a rule, these patients are free of subjective complaints in childhood. They go on to develop early arterial hypertension, however, and this finding plus absent or diminished pulses in the lower half of the body suggest the correct diagnosis1 (see Aorta, p. 28). Both multislice-CT and MRI are useful in the morphological assessment of coarctation. MRI additionally provides functional information on the condition and is therefore preferred in younger patients. Besides evaluating the length and degree of stenosis, it is necessary to determine the pre- and poststenotic diameters, the minimum diameter at the level of the stenosis, and the relationship of the stenosis to the ligamentum arteriosum. The collateral vessels in the chest wall should also be evaluated, as the degree of collateralization correlates with the hemodynamic significance of the stenosis (see Fig. 1.28). Phase-contrast flow measurements can also be performed just distal to the coarctation and at the level of the diaphragm to determine the flow volume through chest-wall collaterals. Determination of the maximum flow velocity just distal to the stenosis permits an estimation of the systolic pressure gradient. MRI can also be used for the morphological and functional evaluation of restenosis following open surgical repair or transluminal intervention (Fig. 13.4). Pseudocoarctation has to be differentiated from coarctation of the aorta. The former is caused by a kink in the aorta at the level of the ligamentum arteriosum. Examination in most of these cases will not indicate significant pressure gradients or a poststenotic increase in flow velocities. Atherosclerotic wall changes occur predominantly in the descending aorta and aortic arch. Atherosclerotic plaques are found less commonly in the ascending aorta but may be a source of cerebral and peripheral emboli. The initial step in the pathogenesis of aortic sclerosis is atherosclerotic thickening of the aortic wall. With the progression of sclerosis, circumscribed plaques form that may bear thrombotic deposits or mobile components that pose a significant embolic risk (Figs. 13.5, 13.6). Aortic sclerosis is classified into five grades of severity: • Grade I: minimal intimal thickening <4 mm • Grade II: pronounced intimal thickening >4 mm Fig. 13.3a, b A 12-year-old girl with preductal coarctation of the aorta after PTA. Hypoplastic posterior aortic arch segment and hypoplastic left subclavian artery. a MIP reconstruction of contrast-enhanced MRA. b MPR of the same dataset. Fig. 13.4a, b A 47-year-old man with postductal coarctation of the aorta and a pressure gradient of 30 mmHg across the stenosis. a Preoperative examination. MIP reconstruction of an MRA dataset. Note the collateral blood supply from chest-wall vessels and the internal mammary arteries (arrows). b Postoperative examination after insertion of a subclavian artery–descending aorta bypass (arrow). Fig. 13.5a, b Detection of a complicated plaque with mobile components (grade V) projecting far into the aortic lumen. a Short-axis TEE view of the midthoracic descending aorta at 35 cm from the incisor teeth. b Corresponding long-axis TEE view. • Grade III: circumscribed atheroma without intraluminal protrusion • Grade IV: atheroma with intraluminal protrusion • Grade V: plaque formation with fissures and mobile material (intima or thrombotic deposits) The normal diameter of the aorta varies with the site of the measurement and the age and sex of the patient. Normal values are 2.3–3 cm for the aortic annulus, 3–3.7 cm for the sinus of Valsalva, and 2.5–3.5 cm for the proximal ascending aorta.2,3 The normal wall thickness is less than 4 mm. The normal values in women are ~10% lower than in men. Besides sex-specific differences, the aortic diameter also depends on physical activity. Persons engaged in strenuous physical labor have larger aortic diameters than more sedentary individuals.2 Aortic diameters should be stated in relation to body surface area. The normal range of values for the ascending aorta is 1.4–2.1 cm/m2 body surface area. The normal range for the descending aorta is 1.0–1.6 cm/m2 body surface area.2,3 With aging, the aortic tube undergoes a continuous, physiological enlargement of ~1–2 mm over a 10-year period. If the aortic diameter is larger than normal (>3.5 cm for the ascending aorta, >3 cm for the descending aorta), it is initially described as aortic ectasia. Dilatation past 4 cm is classified as an aortic aneurysm.2–4 A true aneurysm involves a fusiform (spindle-shaped) or saccular (pouch-shaped) dilatation of all the aortic wall layers, usually as a result of aortic sclerosis. Pseudoaneurysms have at least partially penetrated the vessel wall and are often stabilized only by a fibrous capsule. Pseudoaneurysms frequently have a saccular configuration.4 They may have a traumatic, infectious, or iatrogenic cause or may develop as a complication of penetrating aortic ulcer. Approximately one-third of aortic aneurysms are located in the thoracic aorta, the rest in the abdominal aorta.5 Risk factors for aneurysm formation include the classic atherosclerotic risk factors, especially arterial hypertension, congenital disorders of connective-tissue metabolism in Marfan syndrome or Ehlers–Danlos syndrome, and a bicuspid aortic valve. Fig. 13.6 Contrast-enhanced CT at the level of the main pulmonary trunk demonstrates a calcified plaque with thrombotic deposits in the descending thoracic aorta due to severe atherosclerosis. A pulsation artifact in the ascending aorta mimics an intimal flap (arrow). As a rule, an ascending aortic aneurysm should be treated surgically if its maximum diameter exceeds 5.5 cm or if it enlarges by more than 1 cm per year. If associated changes are present (e. g., severe aortic regurgitation with left ventricular dilatation), it may be appropriate to proceed with surgical replacement of the ascending aorta for aneurysms smaller than 5.5 cm.2,3 Prophylactic aortic replacement is generally recommended earlier in patients with Marfan syndrome, usually when the diameter of the ascending aorta exceeds 4.5 cm.2,3 An aneurysm of the descending aorta requires greater operative trauma, and so surgical treatment is withheld until the aneurysm exceeds 6.5 cm in maximum diameter or enlarges by more than 1 cm per year. Endovascular aortic stent grafting provides a new, minimally invasive alternative to open surgery in the management of these cases.6 Aneurysms smaller than the critical diameter should be followed at regular intervals. The follow-up interval depends on the size of the aneurysm. Aneurysms >5 cm should be followed at relatively close intervals, such as every 3 months.3 If no size change is noted, the follow-up interval may be gradually increased. In all follow-up examinations, the maximum diameter of the aneurysm should be measured at the same site as in previous examinations. • Aortic ectasia: maximum aortic diameter >3.5 cm (ascending aorta) or >3 cm (descending aorta) and <4 cm. • Aortic aneurysm: aortic diameter >4 cm. • Indications for surgical treatment: ascending aorta >5.5 cm (>4.5 cm in Marfan patients), descending aorta >6.5 cm. • Follow-up intervals depend on maximum aneurysm diameter. Aortic aneurysms often remain clinically silent for many years and are detected fortuitously on chest radiographs (Fig. 13.7). Aneurysms of the aortic bulb and proximal ascending aorta can be imaged noninvasively by transthoracic echocardiography. The parasternal long-axis scan can demonstrate the aortic valve and the first 2–4 cm of the ascending aorta and is particularly useful for follow-up examinations (e. g., of the aortic bulb in patients with Marfan syndrome) as it is a rapid, cost-effective procedure that does not require any contrast agent or involve any radiation exposure (Fig. 13.8). Transesophageal echocardiography (TEE) can demonstrate ~5–7 cm of the proximal ascending aorta (Fig. 13.9). Generally it can define the descending aorta from the distal aortic arch (origin of the left subclavian artery) to the thoracoabdominal junction (Fig. 13.10). The distal ascending aorta and the aortic arch itself are obscured by the overlying left main bronchus. This portion of the aorta constitutes a “blind spot” in TEE. Often this procedure is inaccurate in determining maximum aortic diameter because of aortic elongation, which results in tangential sections that overestimate the maximum diameter. CT can image the entire aorta in a matter of seconds. Administration of IV contrast medium should be used in patients evaluated for aortic disease, as it is the only way to provide an adequate assessment of aortic wall pathology (Fig. 13.11). A current serum creatinine should be obtained before the examination owing to the nephrotoxicity of CT contrast medium. It is good practice to image the entire aorta from the annulus to the iliac vessels because of the frequent presence of multilevel disease. Examination of the entire aorta is also important for treatment planning (e. g., identifying the iliac axis as an access site for endovascular aortic stent grafting). Multislice CT technology has made it possible to examine the entire aorta during a single breath hold. Fig. 13.7 PA chest radiograph of a large thoracic aortic aneurysm, which was successfully managed by the previous insertion of an endovascular stent graft. Fig. 13.8 TEE. Parasternal long-axis view shows a conspicuous aneurysm of the aortic bulb in a patient with Marfan syndrome. Fig. 13.9 TEE long-axis view of a normal ascending aorta. Fig. 13.10 TEE image of a type B aortic dissection demonstrates the proximal intimal tear near the origin of the left subclavian artery. Fig. 13.11 a–c Contrast-enhanced ECG-gated multislice CT in a patient with an acute type A dissection, rupture of the aorta, and pericardial effusion. The patient survived emergency surgery despite the aortic rupture. a, b Multiplanar reformatting from the 3D dataset show the entry (arrow), retrograde extension of the dissection down to the origin of the right coronary artery, and a pericardial effusion (star). c Rupture site in the ascending aorta (arrow) with subsequent bleeding into the mediastinum and pericardium. Fig. 13.12a, b Aneurysm of the ascending aorta with consequent enlargement of the aortic annulus and aortic regurgitation. a MIP of contrast-enhanced 3D MRA. b Cine sequence demonstrates the regurgitant jet (arrow). MRI makes it possible to examine the aorta noninvasively without contrast medium or radiation exposure. As in CT, all portions of the aorta should be evaluated. Accurate caliber measurements can be performed on black-blood images. One disadvantage of black-blood imaging is its susceptibility to slow-flow artifacts, which can sometimes mimic thrombus and intimal flaps. For this reason, a bright-blood sequence (e. g., SSFP sequence) should be added so that an accurate assessment can be made (Fig. 13.12). Contrast-enhanced MRA permits three-dimensional visualization of the aorta and has almost completely replaced conventional angiography in aortic investigations. It should be noted, however, that MRA is a luminographic technique and, as such, can image only the perfused lumen; it cannot evaluate the aortic wall or mural thrombosis. For this reason, MRA should always be performed in conjunction with black- or bright-blood sequences. When the diameter of the aorta is enlarged, the maximum diameter should be measured perpendicular to the main direction of blood flow using the centerline method. This can be difficult in the presence of aortic elongation and kinking, and errors may result owing to measurements made in tangential scans. The slow blood-flow velocities in the aneurysm lumen may lead to spontaneous echo contrast as well as mural thrombus formation. The presence of spontaneous echo contrast and thrombi constitutes a significant risk factor for peripheral embolism. Luminographic techniques alone (conventional angiography and MRA) cannot accurately assess the maximum aortic diameter when mural thrombus is present. Often in the aorta it is difficult to distinguish between mural thrombus and intramural hematoma. This can be facilitated by evaluating wall thickening in relation to the aortic intima, which is frequently calcified or thickened in these patients. With mural thrombus, the calcified intima is located on the side opposite the aortic lumen. With intramural hematoma, on the other hand, bleeding into the media shifts the calcified intima toward the vessel lumen (luminal displacement of intimal calcium, Fig. 13.13). Fig. 13.13 Contrast-enhanced CT in a patient with an intramural hematoma of the descending aorta. The intramural hemorrhage has caused luminal displacement of the calcified intima. Fig. 13.14 Schematic drawing of the Svensson classification of the acute aortic syndrome.
Anatomy of the Thoracic Aorta
Congenital Anomalies
Right Descending Aorta
Double Aortic Arch
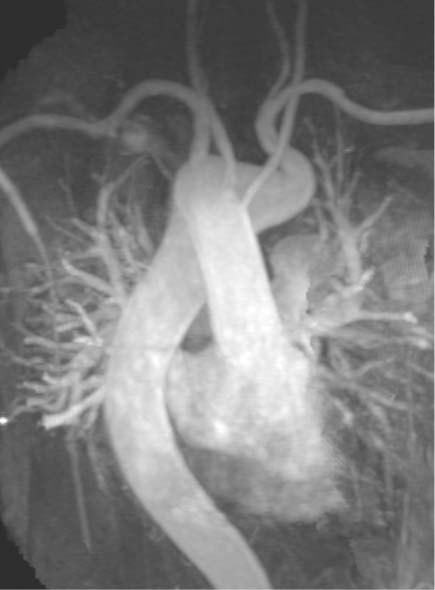
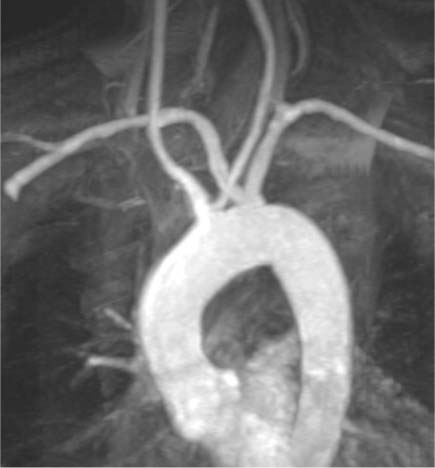
Aortic Arch Anomalies
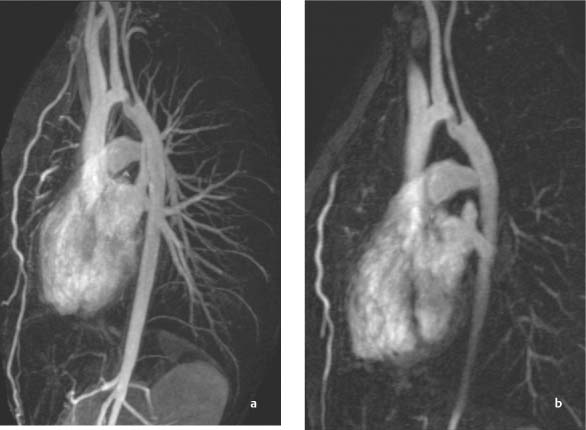
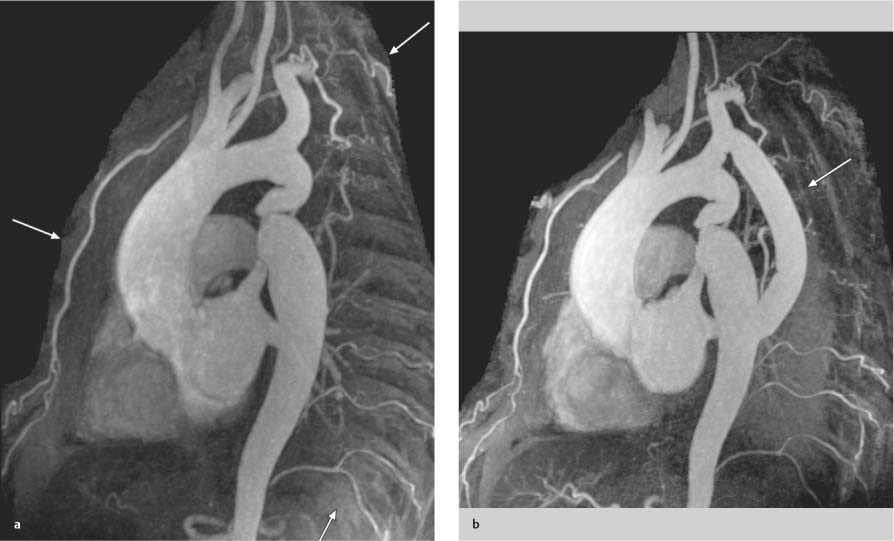
Coarctation of the Aorta
Acquired Aortic Diseases
Degenerative Aortic Diseases
Aortic Sclerosis
Aortic Aneurysm
 Essential Point
Essential Point
Imaging of Aortic Aneurysms
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree