(3)
Department of Radiology, Ege University Hospital, Izmir, Turkey
1.1 Introduction
Pleural diseases are common and represent a significant contribution to the workload of emergency department.
Patients with pleural effusions may be asymptomatic; however, they generally present with symptoms such as pleuritic chest pain or dyspnea.
1.2 Terminology and Clinical Issues
The pleura is composed of visceral and parietal layers. The lungs and interlobar fissures are covered by the visceral pleura. The parietal pleura lines the mediastinum, ribs, and diaphragm. These two layers of pleura are continuous with one another. The area between the two layers is the pleural space. Normally, there is a small amount of fluid within pleural cavity (Collins and Sten 2008; White et al. 2009). Pathological processes may lead to the development of pleural effusions by causing disequilibrium between the rates of pleural fluid formation, pleural permeability, and pleural fluid absorption (Sahn 2008). The pleural fluid can originate from the pleura or may be extrapleural in origin. Pleural effusions may also be seen in the setting of infectious and inflammatory diseases, malignancies, and cardiovascular and systemic diseases (Table 1).
Table 1
Causes of pleural effusion
1. Infectious diseases (bacterial and viral pleurisy, tuberculosis) |
2. Collagen vascular diseases (lupus pleuritis, rheumatoid pleurisy) |
3. Neoplasm (pleural metastases, mesothelioma, leukemia, Non-Hodgkin lymphoma) |
4. Pulmonary embolism |
5. Cardiovascular disease (congestive heart failure, constrictive pericarditis) |
6. Uremic pleuritis |
7. Hypoalbuminemia |
8. Chylothorax |
9. Drug-induced pleural disease |
10. Hypothyroidism |
11. Pancreatitis |
12. Subdiaphragmatic abscess |
13. Hepatic hydrothorax |
14. Trauma |
1.3 Imaging
The chest X-ray (CXR) remains the initial examination of choice in the investigation of pleural disease. Ultrasound (US) is an easily applicable, cheap, and radiation-free method and is most frequently used to assess pleural disease detected on CXR. It can be performed at bedside. In addition to confirmation of pleural effusion, it may be used to guide aspiration or chest-drain insertion (Evans and Gleeson 2004). Computed tomography (CT) is a method that not only detects pleural space but also gives information about the lung parenchyma, mediastinum, and chest wall. CT has ability to determine the presence of pleural fluid loculations and pleural thickening, and it is the best method to differentiate peripheral lung abscess from empyema (King and Thomson 2002; McLoud 1998). Pleural fluid collections and pleural thickening that remain undetermined after CT may undergo magnetic resonance imaging (MRI) (Falaschi et al. 1996).
1.4 Pleural Effusion and Emphyema
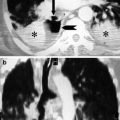
The appearance of pleural effusion depends on the patient’s position at the time of the radiologic examination. Pleural fluid tends to collect along dependent surfaces. In an upright person, fluid collects mainly in the lower pleural space, and accumulation of 200 mL or more of fluid leads to blunting of the lateral costophrenic sulcus. However, it is important to note that the plain film can be normal with up to 500 mL fluid (Blackmore et al. 1996; Collins et al. 1972). As the amount of pleural fluid increases, the diaphragm appears flattened and a homogeneous lower zone opacity with a concave upward border develops. Occasionally, a large amount of pleural fluid may accumulate in the subpulmonic location and may be difficult to diagnose on erect CXR. In case of subpulmonic effusion, the upper edge of the fluid mimics the contour of the diaphragm on the chest radiograph creating an appearance similar to elevated diaphragm (pseudo-diaphragm). However, the peak of the elevated pseudo-diaphragm is more laterally located than normal (Müller 1993) (Figs. 1 and 2). The lateral decubitus view is sensitive in the detection of pleural fluid and can demonstrate as little as 5 mL of fluid. Large amounts of fluid can be missed on a supine radiograph. On a supine radiograph, as fluid accumulates on dependent surfaces, a general increased haziness over the lower pulmonary zones or a density over the apex of hemithorax develops (Fig. 3). Blunting of costophrenic angle may be seen (Müller 1993; Henschke et al. 1989). Loculated effusions can appear confusing on CXR and may be difficult to distinguish from a peripheral lung abscess. The loculated effusion is generally lenticular in shape and does not shift freely in the pleural space with changes in patient position. Incomplete layering may be detected on decubitis films (King and Thomson 2002). Infectious pleural effusions are most commonly associated with pneumonia and are defined as a parapneumonic effusion. Other mechanisms where the pleura may be contaminated by infecting organisms are the rupture of subpleural tuberculous foci or dissemination of infectious particles by the bloodstream. Intra-abdominal infections may reach the pleural space passing through the diaphragm. Penetrating injury to the chest wall or rupture of esophagus can also result in the introduction of organisms into the pleura (Rahman and Davies 2008).
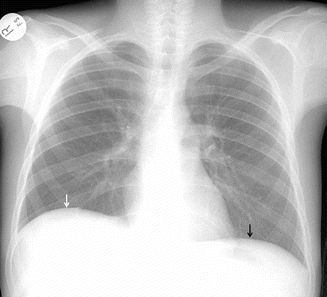

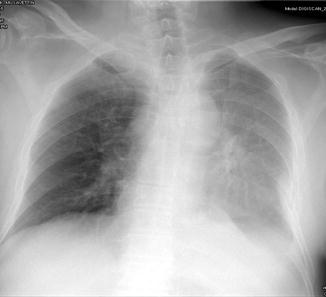

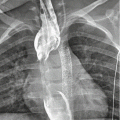
Fig. 1
Posteroanterior chest X-ray of a patient with right subpulmonic pleural effusion. Subpulmonic effusion can simulate elevated diaphragm. The peak of the elevated pseudo-diaphragm is more laterally located than normal (The black arrow demonstrates the peak point of left diaphragm, the white arrow shows the laterally located peak point of left pseudo-diaphragm)

Fig. 2
Right lateral decubitis projection shows right pleural effusion by demonstrating the dependent layering of pleural fluid (white arrows)

Fig. 3
Pleural effusion on left hemithorax on a supine radiograph. The pleural fluid accumulates on dependent surfaces leading to an increased haziness over the left pulmonary zones. There is blunting of left costophrenic angle
Parapneumonic effusions can be separated into three stages.
- 1.
Exudative stage
- 2.
Fibropurulent stage
- 3.
Organizing stage
In the exudative stage of parapneumonic effusion, pneumonic process causes inflammation of the visceral pleura and results in the accumulation pleural fluid. The pleural fluid in this stage is characterized by negative bacterial studies. Pleural thickening may be seen in 50 % of cases at this stage (Rahman and Davies 2008; Kienzl et al. 2012). The fibropurulent stage is caused by pus in the pleural space. The pleural fluid in this stage is infected and is characterized by positive bacterial studies. Progression to emphyema occurs in this stage. At this stage, the split pleura sign is seen on contrast material–enhanced CT images. There is enhancement of the thickened inner visceral and outer parietal pleura, with separation by a collection of pleural fluid (Fig. 4) (Rahman and Davies 2008; Kraus 2007). As the disease progress to organizing stage, fibroblasts grow into the pleural fluid from both the visceral and parietal pleura leading to pleural fibrosis. CT findings at this stage include thickened pleura with multiple loculations. The thickened pleura may calcify. Expansion of extrapleural fat and periosteal changes at adjacent ribs develops. Inability of the lung to expand after tube thoracostomy is seen at this stage (Light 2006). Extensive pleural calcifications may be associated with minimal pleural fluid. This finding is important because residual infection in pleural space may extend to adjacent chest wall and lead to empyema necessitatis or may result in bronchopulmonary fistula formation (Fig. 5a, b) (Collins and Sten 2008). On ultrasound, pleural fluid is most frequently seen as an anechoic or hypoechoic collection.
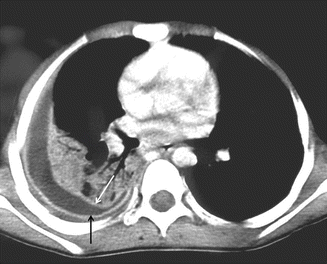
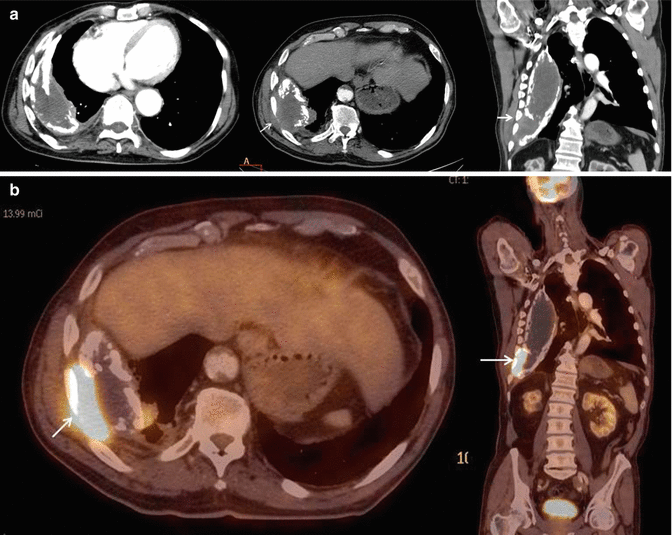

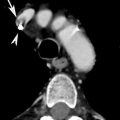
Fig. 4
The split pleura sign in emphyema. Contrast-enhanced axial CT image show enhancement of the thickened inner visceral (white arrow) and outer parietal pleura (black arrow) with separation by a collection of pleural fluid

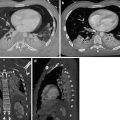
Fig. 5
(a) Empyema necessitatis in a patient with prior history of tuberculosis pleurisy. Contrast-enhanced axial and coronal CT images show thick and calcified pleural layers and associated pleural fluid between the pleural layers. There is extension of pleural infection to the thoracic wall (white arrows). (b). Axial and coronal fused PET-CT images show increased 18F-flurodeoxyglucose (18F-FDG) uptake at level of chest wall extension of pleural infection (white arrow)
The majority of parapneumonic effusions and empyemas are associated with septations, and the pleural fluid at this stage appears hyperechoic on US examination. Pleural thickening is hard to visualize sonographically and if confirmation of this is required, contrast-enhanced CT should be performed. Apparently, there is no definitive correlation between US appearance and the stage of evolution of the effusion (Gleeson 2008).
Sometimes it may be difficult to differentiate between empyema and a pleural-based pulmonary abscess. The pulmonary abscess appears round in shape and forms an acute angle with the chest wall. However, empyemas usually form an obtuse angle with the chest wall. Pulmonary abscesses tend to have thicker walls than empyemas. The “split pleura” sign referring to the separation of enhancing parietal and visceral pleura is seen in empyema and can be used also to differentiate it from an abscess (Gleeson 2008).
1.5 Chylothorax
Chylothorax is defined as an accumulation of chyle in the pleural space most often secondary to thoracic duct injury or malignant invasion. The largest part of the thoracic duct lies in the right hemithorax, and the thoracic duct drains into the junction of the left axillary and internal jugular veins. Most chylous effusions are right sided as expected from this anatomic location. The attenuation of chylous effusions at CT may be low due to the fat content of the fluid. However because of the rich protein content of the chyle, it is generally difficult to differentiate it from other effusions (Radiographic et al. 1997).
1.6 Malignant Effusion
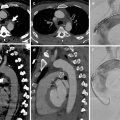
Metastatic seeding of the pleura is seen most commonly in lung, breast, ovarian, and gastrointestinal carcinomas. Primary pleural tumors such as mesothelioma and pleural lymphoma are rare. Certain infiltrative hematologic malignancies (e.g., acute myeloid leukemia) may also involve the pleura (Kienzl et al. 2012; Bonomo et al. 2000; Sahn 1997). Most patients with pleural metastases have large amount of effusions. However, the definitive diagnosis of malignant pleural disease generally requires pleural fluid cytologic examination, pleural biopsy, or even open chest surgery (Collins and Sten 2008; Gleeson 2008). Contrast-enhanced CT is the most commonly performed study in patients with suspected malignant pleural effusion and negative cytology on aspiration. CT signs indicating for both primary and metastatic diseases of pleura include thickening of the mediastinal pleura, parietal pleural thickening of greater than >1 cm, and focal and/or diffuse nodularity of the pleura. Using these criteria for assessment, contrast-enhanced CT has been shown to have a sensitivity of >80 %. CT examination may also give information about associated metastases (Fig. 6). Pleural or diaphragmatic nodules can be detected sonographically in cases with pleural metastases, but CT is better than US for the evaluation of pleural thickening (Evans and Gleeson 2004; Leung et al. 1990). US can be used as a tool to aid thoracentesis. MRI is superior to CT in the detection of small pleural nodules. However, the respiratory and cardiac motion artifacts are the major drawbacks of MRI examination (McLoud 1998; Gleeson 2008). PET and PET-CT have an increasing impact in the diagnosis of malignant pleural tumors. Several studies have mentioned the high accuracy of 18F-FDG PET/CT in differentiating benign and malignant pleural disease, especially in the setting of indeterminate CT findings. Increased pleural FDG uptake usually indicates the presence of pleural metastases (Fig. 7) (Schaffler et al. 2004; Kramer et al. 2004). PET/CT also plays an important role in tumor staging to detect distant metastases and to monitor therapy response. The disadvantage of PET or PET-CT is that they poorly discriminate between infective and malignant causes. Nonspecific FDG uptake can be seen in patients who have undergone prior talc pleurodesis, radiotherapy, intrapleural chemotherapy, etc. (Duysinx et al. 2004; Erasmus et al. 2000). Small volume disease or low grade malignancies such as epitheloid mesothelioma can hardly be detected with PET (Makis et al. 2012).
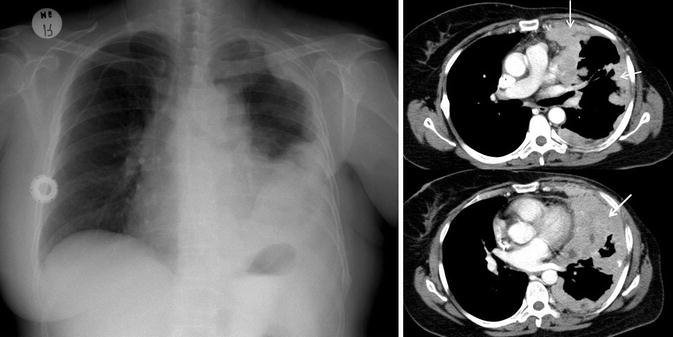
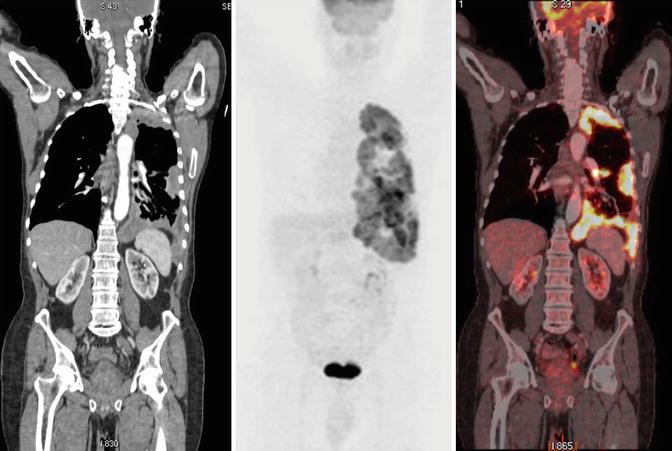

Fig. 6
Pleural metastases in a patient with breast cancer. Posteroanterior chest X-ray and axial contrast-enhanced CT images show left-sided pleural effusion, pleural thickening, and pleural mass lesions (white arrows). There is asymmetry at level of soft tissues due to left mastectomy

Fig. 7
Pleural carcinomatosis. Coronal CT, PET, and PET-CT fusion images show pleural thickening and increased pleural 18F-flurodeoxyglucose (18F-FDG) uptake
1.7 Management and Treatment
Interventional procedures such as thoracentesis, percutaneous drainage, and pleural biopsies are generally performed with the guidance of imaging techniques. Imaging guidance helps identification of the correct placement site and proper insertion of the catheter into the pleural cavity and also helps to avoid complications that can occur during the procedure. The modality of choice depends on several factors such as the location, size, complexity of pleural fluid, local availability of the equipments, and experience of the operator. US can detect small amount of fluid in pleural cavity so it is commonly preferred in bedside percutaneous aspiration and catheter drainage of uncomplicated pleural fluid. CT is generally preferred in case of encapsulated, loculated complex pleural collections. Depending on the characteristics of the pleural fluid such as the amount, pH, glucose levels, bacteriological features, the proper therapeutic method is selected in case of parapneumonic effusion (Light and Rodriguez 1998). Therapeutic methods can vary from therapeutic thoracentesis, insertion of thoracostomy tube, administration of thrombolytics to decortication surgery. Malignant pleural effusion which cannot be controlled by systemic chemotherapy is treated interventionally. The treatment options may be repeated thoracentesis, pleural sclerosis by injection of intrapleural drugs, and long-term pleural catheters (Noukoua Tchuisse et al. 2007).
Reexpansion pulmonary edema (RPE) is a rare but important complication that may occur after treatment of lung collapse caused by pleural effusion. The rapid reexpansion of a chronically collapsed lung, after removal of a large amount of fluid from the pleural space may lead to RPE. The condition appears within 1–24 h after the evacuation. Radiologically, alveolar filling pattern is seen within a few hours of reexpansion of the lung. It is usually unilateral and is detected in those portions of lung that were previously collapsed (Fig. 8) (Tarver et al. 1996). The treatment is generally supportive. As a prevention, limitation of therapeutic thoracentesis to 1000 mL is advised (Light et al. 1980).


Fig. 8
Reexpansion pulmonary edema in a patient with mesothelioma. Axial chest CT image at lung window settings show ground glass areas and air space opacities in left lung after thoracentesis. CT images at mediastinal window settings shows left pleural effusion (white asterisk) and pleural thickening at level of left costophrenic sinus (white arrows)
2 Imaging of Spontaneous Pneumothorax
(4)
Radiology, Emergency Department, La Sapienza University of Rome, Rome, Italy
2.1 Introduction
Pneumothorax is defined as the presence of air in the pleural cavity, between the lung and the parietal pleura.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree