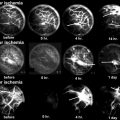
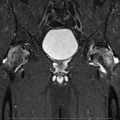
Fig. 31.1
A 44-year-old man had left hip pain for 2 weeks. (a) T1-weighted spin-echo image (TR/TE: 450 ms/20 ms) reveals diffuse, low signal intensity in the left proximal femur. (b) On proton density image (TR/TE: 4,500 ms/15 ms), the lesion changes to a high signal intensity. Hip joint effusion is associated. There is no focal lesion suggestive of osteonecrosis
31.6 Radiographic Findings
In the early stages of disease, plain radiographs of patients with BMES are negative. Demineralization may become evident within 3–6 weeks of onset of symptoms. A focal loss of radiodensity is seen with blurring of the trabecular structure and cortical borders in the femoral head and neck and sometimes extending down to the intertrochanteric region. The radiolucency may remain evident on plain radiographs even after full resolution of symptoms [13]. During the entire course of disease, the bony margins are preserved and no subchondral lesions are evident.
In ON patients, a radiolucent lesion surrounded by a sclerotic rim of reactive bone or a subchondral area of bone collapse or “crescent sign” is typically seen. These findings are not seen in BMES.
31.7 Bone Scan
Bone scanning using TC99 has been useful in detecting pre-radiographic lesions of BMES. Bone scan shows an increased uptake within a few days of symptoms. Generally diffuse uptake throughout the femoral head and into the neck and metaphysis is seen. Bone scan may be used to differentiate BMES from ON, as ON will typically show an area of photopenia (“cold spot”) in the anterior–superior region of the femoral head [13]. The uptake normalizes in accordance with the resolution of edema.
In ON, a cold spot, which represents necrotic potion, is surrounded by a band of hot uptake, which represents the reactive zone.
Currently, bone scanning has been largely replaced by MRI in the diagnosis of BMES, due to its lack of specificity and radiation hazard.
31.8 Histology
Hematopoietic cells are not seen in the marrow. The marrow is filled with eosinophilic fluid and necrotic fat. Fibrosis and dilated vessels are observed in the marrow space. However, most lacunae are filled with osteocytes. Sequestrum of completely dead marrow cells and osteocytes is not formed, and there is no evidence of reparative processes, so called reparative zone of osteonecrosis.
The mineral content in the bone is reduced, which results in visible radiolucency on radiographs. Reactive bone formation with osteoid seams is observed around the trabeculae. Thus, bone formation is increased and there is no evidence of osteoporosis. The term of “transient osteoporosis of the hip” was a misnomer [1].
31.9 Pathophysiologic Relation of ON and BMES
The relationship between BMES and ON remains controversial. Given the self-limiting nature of BMES, some investigators hypothesize that BMES is a separate and distinct condition from ON, while others note that BMES can occasionally progress to ON and therefore suppose that BMES represents an abortive, reversible form of ON [1, 14–16].
Ischemia seems to be the common cause of both ON and BMES. Jones has theorized that intravascular coagulation of the intraosseous microcirculation may be the common pathway to both BMES and ON [14, 15]. However, subsequent events after an ischemic event are different between the two diseases. In BMES the ischemic event is subcritical and reversible. The fatty marrow in BMES specimens can be seen to undergo fibrosis, but the osteocytes remain viable and thus able to recover. In this hypothesis, the determining factor of whether an ischemic event produces BMES or ON is the speed and quality of reperfusion after thrombosis. If the reperfusion is complete, then the patient develops BMES rather than frank necrosis. Angiographic studies of patient with BMES showed arterial dilatation and hyperemia of the proximal femur [17].
Like ON, femoral heads with BMES demonstrate intraosseous hypertension [1, 17]. The bone marrow pressure is usually elevated above 30 mmHg. This intraosseous hypertension is most likely the cause of both the pain and the edema. It is this persistent hypervascularity, which probably causes the demineralization eventually detected on plain radiography. Quantitative radiography confirms a loss of hydroxyapatite without a loss of bone mass (Table 31.1) [1].
Table 31.1
Osteonecrosis and bone marrow edema syndrome
ON | BMES | |
|---|---|---|
Risk factor | Associated in 80 %
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|