MRI method
Authors/study
Major findings
fMRI
Gruesser et al. (2004)
Heightened cue-induced activation in the ACC, striatum, and mPFC predicted subsequent alcohol intake in alcoholics vs. controls
fMRI
Heinz et al. (2007)
Limbic brain activation towards affectively positive stimuli correlated inversely with prospective relapse in alcoholics vs. controls
fMRI
Wrase et al. (2007)
Reduced striatal functional activation during monetary gain anticipation in alcoholics vs. controls, which correlated negatively with craving
Increased striatal activation towards alcohol-associated stimuli in alcoholics vs. controls, which correlated positively with craving severity
fMRI
Myrick et al. (2008)
Anti-craving medication naltrexone and ondansetron reduced alcohol cue-induced striatal activation compared to placebo treatment in alcoholics
The combination treatment decreased craving while viewing alcohol cues in alcoholics
fMRI
Park et al. (2010)
Dysfunctional frontostriatal connectivity predicted abnormal decision-making and higher alcohol craving in male alcohol-dependent patients vs. healthy controls
fMRI + structural MRI
Beck et al. (2012)
Increased alcohol cue-induced mPFC activation in relapsers vs. abstainers
mPFC gray matter loss pronounced in relapsers vs. abstainers
fMRI + structural MRI
Charlet et al. (2013b)
Increased ACC activation towards aversive facial stimuli correlated with less previous lifetime alcohol intake and better treatment outcome in alcoholics vs. healthy controls
Gray matter alterations explained most of the reduced brain activation found in alcoholics
fMRI + structural MRI
Charlet et al. (2013c)
Increased PFC and premotor activations during high working memory load predict low relapse risk in alcoholics
Structural MRI
Schroth et al. (1988)
Reduction in CSF volume after 5 weeks of alcohol abstinence, but no white matter changes
Structural MRI
Di Sclafani et al. (1995)
Age-related ventricular enlargement in alcoholics vs. controls
Structural MRI
Sullivan et al. (1995)
Age-related gray matter volume loss in the anterior hippocampus and temporal cortex; white matter loss and ventricular enlargement in alcoholics vs. controls
Structural MRI
Hommer et al. (1996)
Smaller corpus callosum area among alcoholic women compared to healthy women and to alcoholic men
Structural MRI
Pfefferbaum et al. (1996)
Reduction of the callosal area in chronic alcoholics vs. controls
Structural MRI
Pfefferbaum et al. (1997)
More severe volume deficits in the cortical gray and white matter, sulcal and ventricular enlargement in older vs. younger alcoholics, e.g., in (pre)frontal regions
Structural MRI
Laakso et al. (2000)
Smaller right hippocampal volumes in late-onset type 1 alcoholics and early-onset type 2 alcoholics
Structural MRI
Hommer et al. (2001)
More pronounced reductions of gray and white matter volumes in alcoholic women vs. alcoholic men
DTI
Schulte et al. (2005)
Disruption of corpus callosum microstructure contributed to disturbance in interhemispheric processing in alcoholics
DTI
Pfefferbaum et al. (2006)
Age-related macro- and microstructural white matter disruption of the corpus callosum in alcoholics
DTI
Harris et al. (2008)
Diminished white matter density in the right frontal and cingulum in alcoholics, which was related to working memory performance
PET
Volkow et al. (1996)
Significant reductions in DA D2 receptors (postsynaptic marker) but not in dopamine transporter availability (presynaptic marker) in alcoholics vs. nonalcoholics
PET
Heinz et al. (2005b)
Low striatal DA synthesis capacity and low DA D2/3 receptor availability was linked with high levels of craving in alcoholics
Alcohol craving correlated positively with relapse
PET
Heinz et al. (2005a)
Elevated mu-opiate receptors availability in the ventral striatum, including the NAc in abstinent alcoholics vs. healthy controls, which was associated with higher craving
Remained elevated even after 5 weeks of remeasurement
PET + fMRI
Heinz et al. (2004)
Less DA D2 receptor availability was linked with alcohol craving and increased drug cue-induced striatal activation in detoxified alcoholics vs. controls
SPECT
Heinz et al. (1998b)
Reduced 5-HTT transporter availability in the brainstem area was linked to increased anxiety, depression, and lifetime alcohol consumption in alcoholics
SPECT
Abi-Dargham et al. (1998)
Decreased GABAA receptor density in the ACC, PFC, and cerebellum in abstinent alcoholics
SPECT
Harris et al. (1999)
Decline in cortical/cerebellar perfusion ratio with aging and age at last drink in alcoholics vs. controls
SPECT
Heinz et al. (2000)
Reduced raphe 5-HTT availability in ll-homozygous alcoholics correlated with higher alcohol consumption
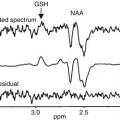
1H-MRS
Martin et al. (1995)
Increases of cerebellar Cho/NAA ratio during the first 3–4 weeks of abstinence; distinct reductions in the Cho/NAA ratio was associated with relapse
1H-MRS
Jagannathan et al. (1996)
Reduced NAA/Cr and NAA/Cho ratios in the frontal lobe, cerebellum, and thalamus in alcoholics vs. controls
1H-MRS
Ende et al. (2005)
Reduced Cho in the frontal white matter and cerebellum, which increased within 3 months of abstinence; trend towards reduced NAA in frontal white matter
1H-MRS
Durazzo et al. (2006)
Partial recovery of regional NAA and Cho in frontal and parietal regions after 1 month of abstinence, which was inversely affected by chronic smoking
1H-MRS
Umhau et al. (2010)
Reduction of the glutamate to Cr ratio across 4 weeks of acamprosate medication in alcoholics
1H-MRS
Hermann et al. (2012)
Increased glutamate absolute concentrations during acute alcohol withdrawal in prefrontocortical regions in alcoholics vs. controls, which normalized within 2 weeks
1H-MRS + structural MRI
Seitz et al. (1999)
Reductions of cerebellar tissue and cerebellar NAA/Cr and CH/Cr ratio in alcoholics vs. controls
1H-MRS + structural MRI
Bendszus et al. (2001)
Normalization of acute withdrawal-related decreased NAA/Cr levels in the frontal lobe and cerebellum and decreased cerebellar CH/Cr ratio in alcoholics after 5 weeks of abstinence
Reduction in the CSF volume within 5 weeks of abstinence in alcoholics
1H-MRS + structural MRI
Bartsch et al. (2007)
Global volumetric brain recovery within 6–7 weeks of sobriety in alcoholics but not in controls, which was partially correlated with increasing cerebellar Cho and frontomesial NAA levels
1H-MRS + structural MRI
Lee et al. (2007)
Increased glutamate to Cr ratio in the ACC was positively related to alcohol consumption during the last month in young abstinent alcoholics
No group differences on gray matter volume found in alcoholics and controls
19.3 Drug Dependence Other than Alcoholism
All psychotropic drugs of abuse are characterized by a dysfunctional responsiveness of the brain reward system and share adaptational mechanisms also found in alcohol dependence. Hence, drug and alcohol dependence is characterized by increased tolerance to excessive drug intake due to homeostatic counteradaptations such as reductions in neuroreceptors stimulated by drugs of abuse, and withdrawal symptoms appear as an expression of impaired homeostasis between excitatory and inhibitory neurotransmitter systems once drug intake is suddenly stopped during withdrawal (Koob 2003; Heinz et al. 2009a). Likewise, all drugs of abuse stimulate DA release, and this activating effect does not appear to habituate, resulting in counteradaptive downregulations of DA neurotransmission and impaired effects of nondrug rewards, thus biasing the individual towards urging for the relative strong effects of drug consumption (Heinz et al. 2009a, b).
19.3.1 Heroin/Opiates
Opiates suppress the inhibitory effect of GABAergic neurons on dopaminergic neurons in the ventral tegmental area, resulting in an increased activity of DA neurotransmission in the ventral striatum (Kreek 2008). Opiates also strongly impact on opiate receptors and inhibit noradrenergic neurons in the locus coeruleus (Kreek 2008). The thus suppressed noradrenergic activity results in clinically manifest symptoms of opiate intoxication such as bradycardia, drowsiness, and slow respiration. This opioidergic inhibition of noradrenergic neurons in the locus coeruleus results in counteradaptive upregulation of noradrenergic neurotransmission, resulting in a new homeostatic balance between noradrenergic neuroadaptation and opioidergic inhibition as long as opiate intake is maintained on a relatively regular basis. However, when opioidergic inhibition of noradrenergic neurotransmission is suddenly interrupted during abstinence, noradrenergic hyperactivity occurs with typical physical and affective withdrawal symptoms, e.g., tachycardia, transpiration, restlessness, sleep disorders, and anxiety (Kosten and George 2002). Because of heroin’s short half-life period of 6 h, dependent subjects need to consume heroin several times a day in order to avoid aversive withdrawal symptoms, thus negatively reinforcing drug intake via the avoidance of a highly unpleasant state. Interestingly, withdrawal symptoms can also occur as a consequence of context-conditioned learning: Siegel (1983) observed that counteradaptive mechanisms lead to withdrawal symptoms as soon as rats used to opiate application in a certain context (cage) are left in this environment without opiate supply. Context-dependent cues thus seem to activate, e.g., noradrenergic upregulation, resulting in hyper-excitation once opiate application does not occur as expected. If on the other hand usual opiate doses were applied in an unfamiliar context, e.g., an unknown cage, animals used to a certain opiate dose displayed symptoms of opiate intoxication and even died, indicating that in the unknown context, their organisms failed to actively prepare for renewed intoxication via context-conditioned neuroadaptational processes (Siegel 1983). In humans, comparable conditioned effects of chronic drug intake may manifest as perception biases towards drug-associated stimuli and memory including related emotions and modification of (aversive) emotional states. For instance, Lubman and colleagues observed that long-term heroin users vs. healthy controls displayed altered processing of drug-related (e.g., pictures of drug preparation and injection) and pleasant pictures (e.g., pictures of food, erotic nudes): positive pictures, contrary to drug-related pictures, elicited less physiological responses and lower self-reported arousal in drug-dependent subjects. Further, low valence ratings of pleasant pictures predicted consistently regular heroin use (at least once a week) at a median of a 6-month follow-up assessment (Lubman et al. 2009). Like in alcohol dependence, prolonged abstinence allows for neurofunctional recovery, hypothetically reflected in decreased salience of drug-associated cues, thereby, reducing the risk of relapses. Indeed, an fMRI study indicated that following long-term compared to short heroin abstinence, subjects displayed decreased neural responses to heroin-related cues in brain regions subserving visual sensory processing, attention, memory, and action planning (Lou et al. 2012).
19.3.2 Cocaine and Other Psychostimulants
The strong effects of cocaine on the DA system can be demonstrated by the fact that the consumption of this substance discharges about 400 % of the dopamine doses normally released by natural reinforcers, such as food or sexual activities (Hurd et al. 1990). These massive effects lead to a counteradaptive reduction in (striatal) DA D2 receptor availability in cocaine-dependent subjects (Volkow et al. 1993), and this striatal downregulation predicted reduced functional responses to a non-drug-related reinforcer (monetary reward) in the thalamus, a brain region that is implicated in DA-modulated conditioned responses and reward expectation (Asensio et al. 2010). However, in this study also a low DA D2 receptor availability in the dorsal and ventral striatum was found to be correlated with high responsiveness of the dorsal superior medial frontal gyrus (Brodmann area 6/8/32), an area that is crucially involved in behavioral monitoring. Asensio and coworkers assumed that cocaine-addicted individuals who displayed lower ventral striatal DA D2 receptor availability are also the ones who possibly need to compensate particularly for their reduced sensitivity to monetary reinforcement (Goldstein et al. 2007) or for their increased impulsivity (Asensio et al. 2010). Other fMRI-based study revealed reduced brain activation elicited by naturally reinforcing visual stimuli, e.g., pictures with erotic content, while cocaine-associated cues which triggered craving elicited increased functional responses throughout the brain including prefrontal, limbic, and parietal regions (Garavan et al. 2000). It has been hypothesized that prefrontal brain activation elicited by drug-related stimuli impairs executive function and behavior control (Beck et al. 2012). Indeed, a prospective fMRI study by Paulus et al. (2005) observed that decreased activation of the PFC during a decision-making task was associated with the subsequent relapse risk in methamphetamine-dependent patients.
19.4 Non-substance-related Addiction
Another class of addictions are the so-called non-substance-related or behavioral addictions, e.g., pathological gambling. Referring to ICD-10, pathological gambling is subsumed in the chapter “Disorders of Adult Personality and Behavior” in the category “habit and impulse disorders” (Dilling et al. 2000), whereas in DSM-IV, pathological gambling is classified as an “impulse-control disorder” (Saß et al. 2003).
Looking at the criteria, pathological gambling and substance-related disorders share a variety of symptoms (Grüsser and Thalemann 2006; Potenza 2006):
An appetitive urge or strong craving state prior to the engagement in the behavior
Preoccupation with gambling (e.g., preoccupied with reliving past gambling experiences, handicapping or planning the next venture, or thinking of ways to get money with which to gamble)
Need to gamble with increasing amounts of money in order to achieve the desired excitement
Repeated unsuccessful efforts to control, cut back, or stop gambling
Continued engagement in the behavior despite adverse consequences: the person has jeopardized or lost a significant relationship, job, or educational or career opportunity because of gambling
Also imaging data suggest a closer relationship between pathological gambling and substance use disorders than between pathological gambling and obsessive-compulsive disorder (Potenza 2008). For example, neurobiological studies investigated cue reactivity processes in behavioral addictions, i.e., when subjects are confronted with stimuli closely related to gambling. Crockford and associates (2005) observed that pathological gamblers exhibited significantly greater activity in the right dorsolateral prefrontal cortex (DLPFC), including the inferior and medial frontal gyri, the right parahippocampal gyrus, and the left occipital cortex, including the fusiform gyrus, during visual presentations of gambling-related video alternating with video of nature scenes. They moreover reported a significant increase in craving for gambling after the study.
Other studies suggested that there is also a reduction in the sensitivity of the reward system in behavioral addictions that resembles reward system dysfunction in substance dependence: Reuter and colleagues (2005) used fMRI to investigate pathological gamblers and healthy volunteers during a game. They observed a reduction of ventral striatal and ventromedial prefrontal activation in pathological gamblers, which was negatively correlated with gambling severity, linking hypoactivation of these areas to disease severity (Reuter et al. 2005). In another study among pathological gamblers, de Ruiter et al. (2009) showed a diminished reward and punishment sensitivity as indicated by hypoactivation of the ventrolateral PFC when money was gained and lost.
As described earlier, alcohol dependence is associated with smaller gray matter volumes in a variety of cortical and subcortical brain regions.Comparably van Holst et al. (2012) hypothesized that there might be structural abnormalities in pathological gamblers, because the same cognitive impairments have been found in patients suffering from problem gambling behavior and from substance dependence. However, in contrast to alcohol, gambling behavior does not expose the brain to toxic agents. Using voxel-based morphometry, van Holst et al. assessed gray matter volumes in treatment-seeking problem gamblers, subjects with alcohol use disorders, and healthy volunteers. They replicated previous findings of smaller gray matter volumes in subjects with alcohol use disorders, but did not observe significant morphological brain abnormalities in problem gamblers. Moreover, Joutsa et al. (2011) investigated possible changes in regional brain gray and white matter volumes as well as white matter integrity in pathological gamblers compared to healthy controls; again there was no volumetric difference in gray matter as well as in white matter between pathological gamblers and controls. However, in pathological gamblers, Joutsa and colleagues observed lower white matter integrity in multiple brain regions including the corpus callosum, the cingulum, the superior and inferior longitudinal fascicle, the anterior thalamic radiation, and the inferior fronto-occipital fascicle, which require replication in further studies (e.g., Lane et al. 2010).