© Springer International Publishing Switzerland 2015
Patricia M. Carrascosa, Ricardo C. Cury, Mario J. García and Jonathon A. Leipsic (eds.)Dual-Energy CT in Cardiovascular Imaging10.1007/978-3-319-21227-2_11. Dual Energy CT
(1)
CT and MR Department, Diagnóstico Maipú, Av. Maipú 1668, Vicente Lopez, Buenos Aires, 1602, Argentina
(2)
Cardiology Department, Montefiore Einstein Center for Heart and Vascular Care Center, New York, NY, USA
(3)
Department of Radiology, Miami Cardiac and Vascular Institute and Baptist Health of South Florida, Miami, FL, USA
(4)
Department of Radiology, Providence Health Care, Vancouver, BC, Canada
Abstract
Dual Energy CT (DECT) represents the newest significant advancement in the computed tomography field.
Alvarez and Macovski were among the first to describe its potential applications. Although the implementation was delayed primarily owing to technical difficulties since 2006, improvements in temporal resolution and co-registration have made a dramatic development in DECT technology.
Currently there are three technical approaches of DECT available. All of these alternative hardware configurations acquire studies with different X-ray spectra assigning tissues characterization according to their chemical composition and thus entering the field of functional analysis.
Outside the cardiovascular system, DECT has more advanced developments in several clinical scenarios.
The role of DECT in the cardiovascular field has become more common lately.
In summary this textbook will be focused in the rapidly developing and promising novel applications of DECT in the cardiovascular field.
Keywords
Dual energy CT (DECT)Cardiovascular applications of dual energy CTMonochromatic role of DECT in cardiacMaterial decomposition in perfusion evaluationSince Sir Godfrey Hounsfield and Allan Cormack invented the computed tomography (CT) scanner in the late 1960s, the technology has rapidly advanced from the single-slice to advanced ultra-fast multi-row detector systems. Dual Energy CT (DECT) represents the newest significant advancement in the field. First investigated in the late 1970s, at the time the organ of interest required have to be scanned twice [1]. Alvarez and Macovski [2] were among the first to describe its potential applications, although the implementation was delayed primarily owing to technical difficulties such as low spatial resolution, suboptimal signal to noise ratio, and high radiation doses.
With the advent of the first dual source dual energy capable scanner in 2006 there has been a relative explosion in interest and integration of DECT into clinical practice. Improvements in temporal resolution together with new methods for reducing radiation doses and improving co-registration have made a dramatic improvement in DECT technology [3]. Recently, new CT devices capable of generating two X-ray beams of low and high energy have been introduced into clinical practice. Currently there are three technical approaches of DECT available:
Dual-source CT scanner with 80 (100) kVp and 140 kVp tubes (Siemens Medical Solutions)
Dual-layer multi-detector scanner with acquisition at 120 or 140 kVp (Philips Healthcare);
CT unit with one rapid kVp switching source and new detector based on gemstone scintillator materials (GE Healthcare).
All of these alternative hardware configurations acquire studies with different X-ray spectra assigning tissues characterization according to their chemical composition and thus entering the field of functional analysis [4]. DECT offers a substantial shifting in the diagnostic approach as it allows analyzing the information according to the chemical composition of the tissues in order to detect pathological findings even in the absence of morphological or densitometrical abnormalities, something previously unexpected to be done by CT. For this reason DECT opens new horizons in image analysis. Dual energy techniques offer a potential to distinguish between substances such as iodine, calcium, uric acid crystals, gadolinium, and xenon, among others. All these substances have high atomic number or “Z” and show different behavior in the spectrum range allowing better tissue characterization. Other materials such as water, fat, and soft tissues have similar attenuation at different energies. Thereby, the elements that work best with DECT are those with high atomic number.
Many scientific publications described the potential of DECT to detect and characterize pathologic conditions. Most articles describe general applications with focus in the brain, neck, thorax, abdomen, and musculoskeletal systems. The role of DECT in the cardiovascular field has become more common lately due recent advancements that have enabled the integration of dual energy applications with ECG gated techniques. Therefore, this book attempts to bring together the main clinical indications of DECT in the cardiovascular field.
Outside the cardiovascular system, DECT has more advanced developments in several clinical scenarios. Urinary stones composition is the most known application. DECT can determine the stone component (uric acid, calcium, Brushita, apatite) and therefore aids the choice of the best therapeutic alternative for a particular patient [5].
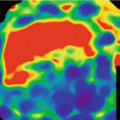
DECT has great impact on the study of solid lesions in different organs because it allows a fast, easy, safe and reliable discrimination between cysts and solid masses without densities measurement. It is known that malignant lesions have an iodine uptake above 20 HU, which is difficult to measure by SECT in certain cases. With iodine DECT map, lesions can be assessed accurately, reducing significantly the interpretation time, avoiding the pseudo-enhancing phenomenon, and decreasing the number of masses with indeterminate diagnosis [6]. DECT allows quantifying the amount of iodine in a mass thus defining the degree of tumor vascularization and evaluating the potential response to treatment with anti-angiogenic drugs. Consequently, DECT has the capability of distinguish between viable tumor and necrosis.
In relation to tissue perfusion DECT has demonstrated excellent results for the diagnosis of pulmonary embolism confirming hypo-perfusion pulmonary defects associated to vascular emboli [7].
The main musculoskeletal application is the identification of uric acid in soft tissues of patients with gout. However, there are new potential applications in this field such as indemnity evaluation of tendons and ligaments [8].
The usefulness of DECT in the cardiovascular field appeared later due to the need for ECG gating and improvement in temporal resolution.
As technology advances, DECT has the possibility of analyzing the information with different approaches. Monochromatic evaluation and material decomposition are the cornerstones of DECT [9].
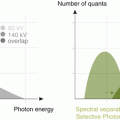
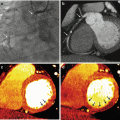
Monochromatic evaluation allows to studying the tissues at different energetic levels, showing their behavior in different energy spectrum ranging from 40 to 140 kVp [10, 11]. Lower levels show higher contrast differentiation for tissues with high Z number such as iodine, calcium and gadolinium among others. These levels are limited by an increased image noise. For that reason, it is necessary to use iterative reconstruction in the levels that DECT can support (up to 60 keV) so as to compensate the noise. On the other hand, high energetic levels reduce the contrast and can even subtract iodine from the images. These are called virtual non-contrast images (VNC) [12]. Low energetic levels (40–60 kev) at monochromatic evaluation show significant intraluminal enhancement thus allowing to reducing the total amount of contrast media that is required for a vascular CT study up to 50 %. There are many studies showing this useful application for the evaluation of different vascular territories such as the pulmonary arteries, coronaries and others [13]. In addition, DECT could play a role in patients with aortic stent grafting, where it could improve the diagnostic accuracy for the identification of endoleaks. In some settings, single-energy CT (SECT) can have difficulties in the identification of these complications when the leak has low attenuation at conventional SECT tube potential. On the contrary, DECT with low energetic levels can help in identifying small or low enhancement endoleaks and potentially result in a precise diagnosis [14].
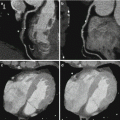
Significant reductions of total contrast volume may allow to perform DECT studies in patients with borderline renal function that otherwise could not be studied. Also, low energetic levels could help in differentiation of fixed defects in the myocardium, making the identification of scar or necrotic segments easily to identify. The potential role of DECT for the assessment of myocardial viability and the detection of myocardial infarction has been evaluated in a limited number of patients [15–17].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree